
An organic compound with the molecular formula ${C_8}{H_8}O$ forms 2,4 – DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. The organic compound is:
(A) 2-ethylbenzaldehyde
(B) 2-methylbenzaldehyde
(C) acetophenone
(D) 3-methylbenzaldehyde
(E) phenylacetaldehyde
Answer
598.2k+ views
Hint: It is a carbonyl compound with no active hydrogen atoms or α-hydrogens. It has benzene parent carbon chain and oxidation product of 1,2-benzenedicarboxylic acid suggests that it has 2 substituents at 1 and 2 positions.
Complete step by step answer:
The molecular formula of the given compound is: ${C_8}{H_8}O$.
-Since the organic compound gives a 2,4 – DNP derivative, it means that it is a carbonyl compound because 2,4 – dinitrophenyl hydrazine (Brady’s reagent) reacts only with aldehydes and ketones to give a coloured precipitate.
Now we need to know whether the carbonyl group is of ketone or of aldehyde.
-This compound also reduces Tollen’s reagent and undergoes Cannizzaro reaction.
Tollen’s reagent is a solution of: silver nitrate ($AgN{O_3}$) and ammonia ($N{H_3}$) and is used for the detection of aldehydes. This reagent converts aldehydes to carboxylic acids. The general form of reaction by Tollen’s reagent is:
$R - COH + 2A{g^ + } + 2O{H^ - } \to R - COOH + 2Ag + {H_2}O$
Silver mirror-like surfaces on the test tube indicate the presence of an aldehydic group. Due to this reason, Tollen’s test is also known as the silver mirror test.
Cannizzaro reaction: It is a reaction to check for the presence of aldehydes which do not have any active hydrogen or α-hydrogen. Here the aldehyde reacts with a strong base to undergo a redox (oxidation-reduction) reaction. This converts aldehydes into alcohol and carboxylic acid. The general form of Cannizzaro reaction is:
$2R - CHO\xrightarrow[{{H_3}{O^ + }}]{{NaOH}}R - C{H_2}OH + R - COOH$
So, reducing Tollen’s reagent and giving Cannizzaro reaction means that there are no α-hydrogen and the compound has an aldehydic group.
So, this tells us that the compound will be a benzene ring with 2 substituents: an aldehydic group ($ - CHO$) and a methyl group ($ - C{H_3}$).
-Since vigorous oxidation of this compound gives 1,2-benzenedicarboxylic acid, so it proves that the aldehydic group ($ - CHO$) and the methyl group ($ - C{H_3}$) are present at successive positions.
So, the compound will be: 2-methylbenzaldehyde and its structure will be:
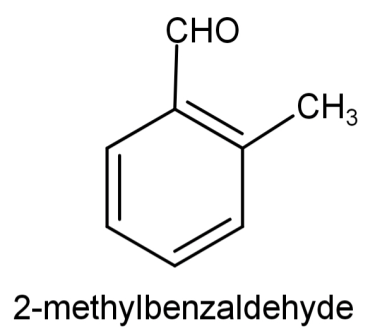
And the reactions involved will be:
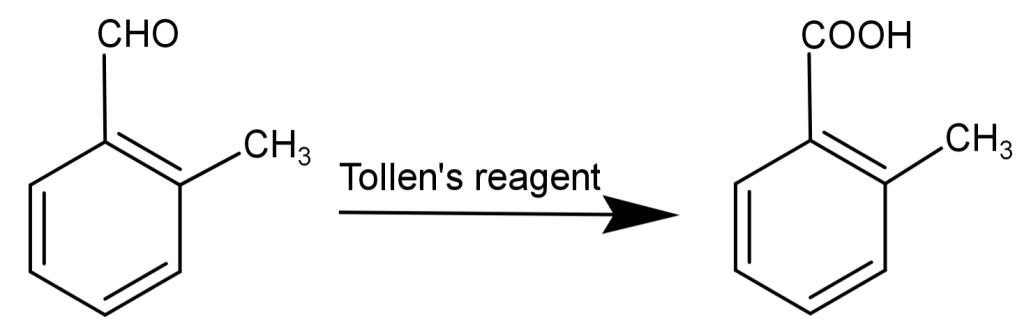
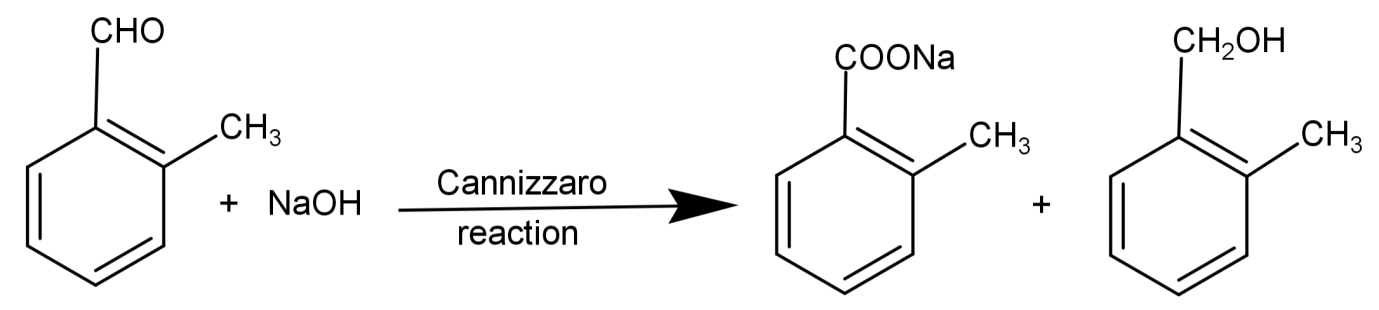
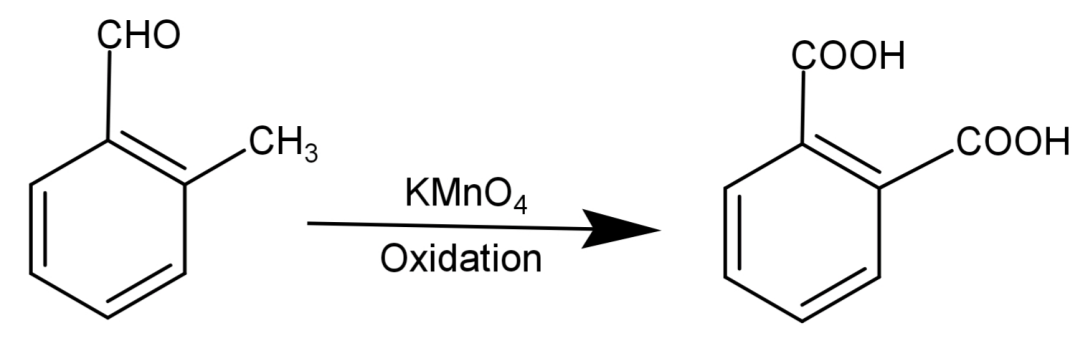
So, the correct answer is “Option B”.
Note:Active hydrogens or α-hydrogens are those H atoms which are attached to the carbon atom just adjacent to the functional group carbon or to the α-carbon atom. Also the alpha hydrogen of carbonyl groups are highly acidic due to stability of the anion which will be formed once the hydrogen atom is removed.
Complete step by step answer:
The molecular formula of the given compound is: ${C_8}{H_8}O$.
-Since the organic compound gives a 2,4 – DNP derivative, it means that it is a carbonyl compound because 2,4 – dinitrophenyl hydrazine (Brady’s reagent) reacts only with aldehydes and ketones to give a coloured precipitate.
Now we need to know whether the carbonyl group is of ketone or of aldehyde.
-This compound also reduces Tollen’s reagent and undergoes Cannizzaro reaction.
Tollen’s reagent is a solution of: silver nitrate ($AgN{O_3}$) and ammonia ($N{H_3}$) and is used for the detection of aldehydes. This reagent converts aldehydes to carboxylic acids. The general form of reaction by Tollen’s reagent is:
$R - COH + 2A{g^ + } + 2O{H^ - } \to R - COOH + 2Ag + {H_2}O$
Silver mirror-like surfaces on the test tube indicate the presence of an aldehydic group. Due to this reason, Tollen’s test is also known as the silver mirror test.
Cannizzaro reaction: It is a reaction to check for the presence of aldehydes which do not have any active hydrogen or α-hydrogen. Here the aldehyde reacts with a strong base to undergo a redox (oxidation-reduction) reaction. This converts aldehydes into alcohol and carboxylic acid. The general form of Cannizzaro reaction is:
$2R - CHO\xrightarrow[{{H_3}{O^ + }}]{{NaOH}}R - C{H_2}OH + R - COOH$
So, reducing Tollen’s reagent and giving Cannizzaro reaction means that there are no α-hydrogen and the compound has an aldehydic group.
So, this tells us that the compound will be a benzene ring with 2 substituents: an aldehydic group ($ - CHO$) and a methyl group ($ - C{H_3}$).
-Since vigorous oxidation of this compound gives 1,2-benzenedicarboxylic acid, so it proves that the aldehydic group ($ - CHO$) and the methyl group ($ - C{H_3}$) are present at successive positions.
So, the compound will be: 2-methylbenzaldehyde and its structure will be:

And the reactions involved will be:



So, the correct answer is “Option B”.
Note:Active hydrogens or α-hydrogens are those H atoms which are attached to the carbon atom just adjacent to the functional group carbon or to the α-carbon atom. Also the alpha hydrogen of carbonyl groups are highly acidic due to stability of the anion which will be formed once the hydrogen atom is removed.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




