
An organic compound with molecular formula ${C_6}{H_{12}}$ upon ozonolysis gave only acetone as the product. The compound is:
A.2,3-dimethyl-1-butene
B. 3-hexene
C.2-hexene
D.2,3-dimethyl-2-butene
E.3-methyl-1-pentene
Answer
581.4k+ views
Hint:Ozonolysis reaction is used to identify the position of double bond in alkenes or other unsaturated compounds. In an ozonolysis reaction, the substrate reacts with an ozone molecule to give ozonide which when further treated with zinc gives the final product. The final product can be a ketone or aldehyde.
Complete step by step answer:
Definition: Ozonolysis is a reaction in which an ozone molecule is added to the unsaturated bond in a molecule to form ozonide and then this ozonide is cleaved or broken into smaller molecules. This reaction is usually carried out with alkenes.
In ozonolysis the carbon-carbon bond in alkenes or alkynes is replaced by a carbonyl group.
The molecular formula ${C_6}{H_{12}}$ indicates that the compound is alkene. ${C_n}{H_{2n + 2}}$ is the general molecular formula for alkene where $n$is the number of carbon atoms.
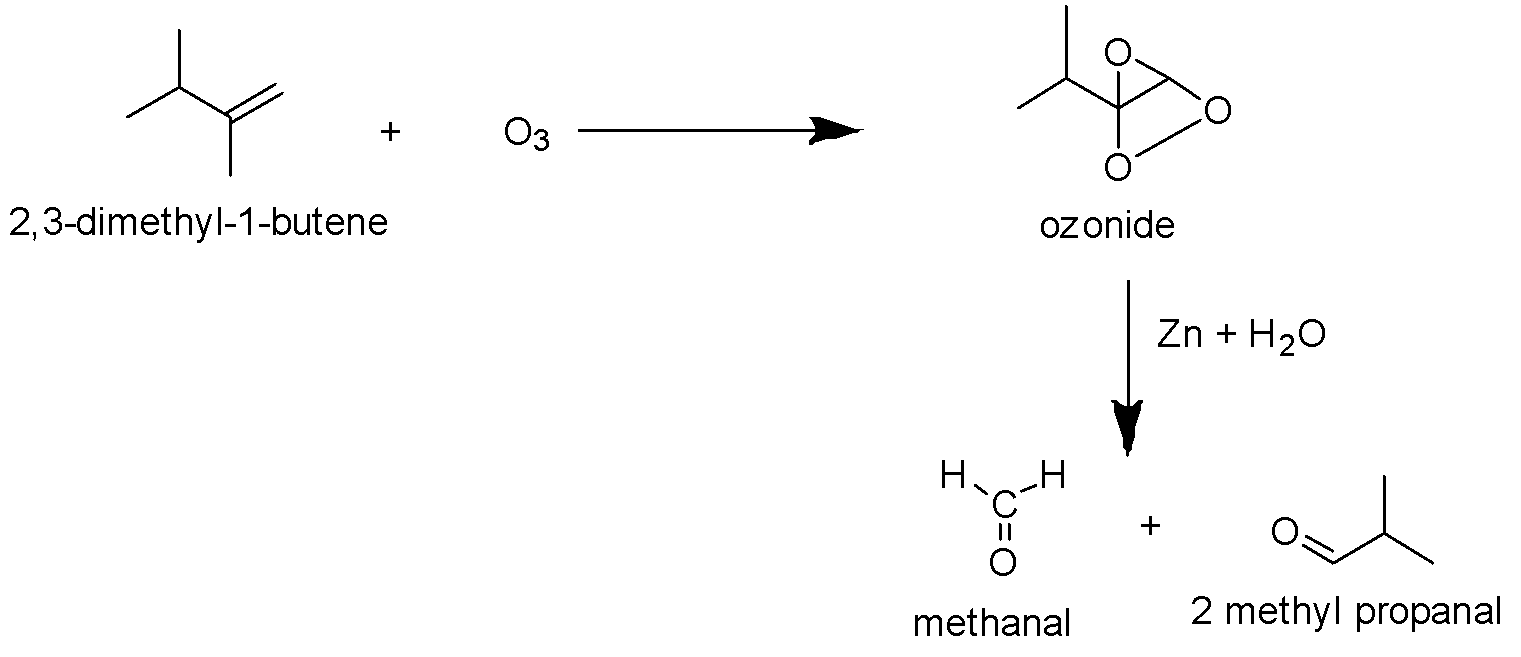
Now let us find out the products of the ozonolysis reaction of each given molecule. The ozonolysis reaction of 2,3-dimethyl-1-butene is as follows.It gives methanal and 2-methylpropanal as the major product.
Now, similarly try to identify the product of ozonolysis of other molecules too.
The ozonolysis of 3-hexene gives two molecules of propanal as the major product.

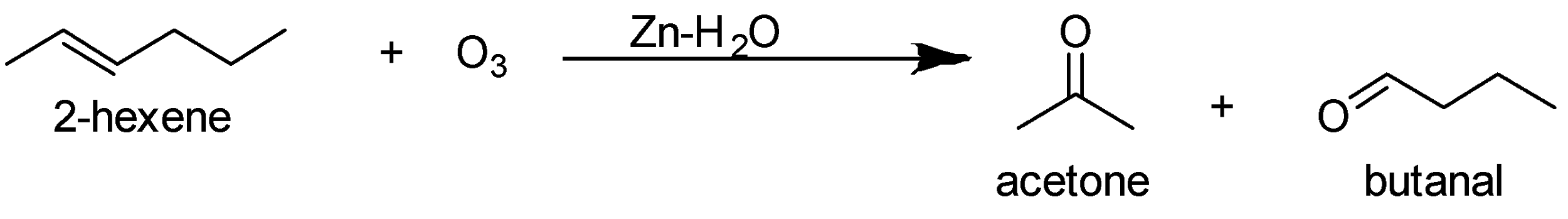
The ozonolysis of 2-hexene gives acetone and butanal as the major product.
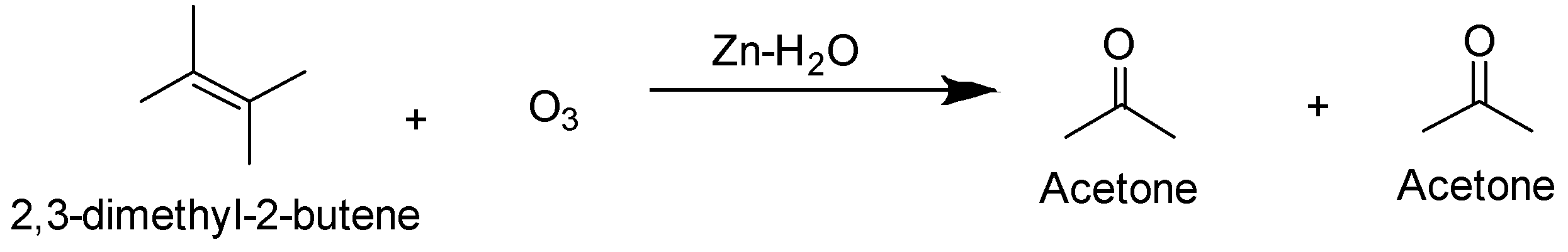
The ozonolysis of 2,3-dimethyl-2-butene gives acetone as the only product.
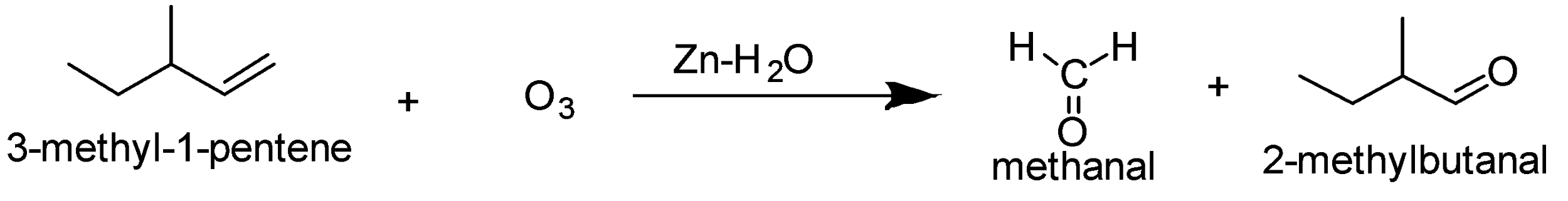
The ozonolysis of 3-methyl-1-pentene gives methanal and 2-methylbutanal as the product.
So the correct answer is option (D) 2,3-dimethyl-2-butene.
Additional information:
-The reagents required for ozonolysis are ${O_{{3_{}}}}\,and\,Zn - {H_2}O$ .
-Unsaturated compounds like alkanes, alkenes undergo ozonolysis reactions.
Note:
-Zinc prevents the molecule from further forming a bond with oxygen molecules. It prevents further oxidation.
-There is a trick for identifying the ozonolysis reaction product. Cleave the carbon-carbon bond and convert the end carbon atoms of the double bond into carbonyl groups.
Complete step by step answer:
Definition: Ozonolysis is a reaction in which an ozone molecule is added to the unsaturated bond in a molecule to form ozonide and then this ozonide is cleaved or broken into smaller molecules. This reaction is usually carried out with alkenes.
In ozonolysis the carbon-carbon bond in alkenes or alkynes is replaced by a carbonyl group.
The molecular formula ${C_6}{H_{12}}$ indicates that the compound is alkene. ${C_n}{H_{2n + 2}}$ is the general molecular formula for alkene where $n$is the number of carbon atoms.
Now let us find out the products of the ozonolysis reaction of each given molecule. The ozonolysis reaction of 2,3-dimethyl-1-butene is as follows.It gives methanal and 2-methylpropanal as the major product.

Now, similarly try to identify the product of ozonolysis of other molecules too.
The ozonolysis of 3-hexene gives two molecules of propanal as the major product.

The ozonolysis of 2-hexene gives acetone and butanal as the major product.

The ozonolysis of 2,3-dimethyl-2-butene gives acetone as the only product.

The ozonolysis of 3-methyl-1-pentene gives methanal and 2-methylbutanal as the product.

So the correct answer is option (D) 2,3-dimethyl-2-butene.
Additional information:
-The reagents required for ozonolysis are ${O_{{3_{}}}}\,and\,Zn - {H_2}O$ .
-Unsaturated compounds like alkanes, alkenes undergo ozonolysis reactions.
Note:
-Zinc prevents the molecule from further forming a bond with oxygen molecules. It prevents further oxidation.
-There is a trick for identifying the ozonolysis reaction product. Cleave the carbon-carbon bond and convert the end carbon atoms of the double bond into carbonyl groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




