
An organic compound contains $66\% $ carbon, $13.3\% $ hydrogen and the remaining is oxygen. Its vapour density is $37$ . The possible number of isomers of all types of the compound is:
A.$6$
B.$7$
C.$5$
D.$8$
Answer
562.5k+ views
Hint: In this question, in order to find the number of possible isotopes, first we need to find the molecular formula of the organic compound. Molecular formulas can be easily found using the percentage mass of elements in compounds and its molecular mass.
Complete step by step answer:
Given, vapour density of the compound is $37$. Molecular mass can be find out from vapour density using the formula,
Molecular mass = Vapour density $ \times 2$
Hence molecular mass of given compound is,
Molecular mass $ = 37 \times 2 = 74g/mol$
Given the organic compound contains $66\% $ carbon, $13.3\% $ hydrogen and the remaining is oxygen. Let x be the weight of carbon in the compound. Then we can write,
$
\dfrac{x}{{74}} \times 100 = 66 \\
x = \dfrac{{66 \times 74}}{{100}} = 48.84g \\
$
The compound contains $48.84g$ of carbon. Atomic mass of one carbon atom is $12g$. Hence number of carbons in the compound will be,
$\dfrac{{48.84}}{{12}} = 4.07$
We can round it to $4$ .
Similarly we can find out the number of hydrogens and oxygens present in the molecule.
Let y be the weight of hydrogens in the compound. Then we can write,
$
\dfrac{y}{{74}} \times 100 = 13.3 \\
y = \dfrac{{13.3 \times 74}}{{100}} = 9.842g \\
$
The compound contains $9.842g$ of hydrogen. Atomic mass of a hydrogen atom is $1g$. Hence number of hydrogens in the compound will be,
$\dfrac{{9.842}}{1} = 9.842$
We can round it to $10$ .
Let z be the weight of oxygen in the compound. The percentage of oxygen in the compound will be,
$20.7\% $ . Then we can write,
$
\dfrac{z}{{74}} \times 100 = 20.7 \\
z = \dfrac{{20.7 \times 74}}{{100}} = 15.318g \\
$
The compound contains $15.318g$ of oxygen. Atomic mass of an oxygen atom is $16g$. Hence number of oxygens in the compound will be,
$\dfrac{{15.318}}{{16}} = 0.9574$
We can round it to $1$ .
Hence the molecular formula of the compound is ${C_4}{H_{10}}O$ . Let us draw all the possible structures with this molecular formula. The possible structures are,
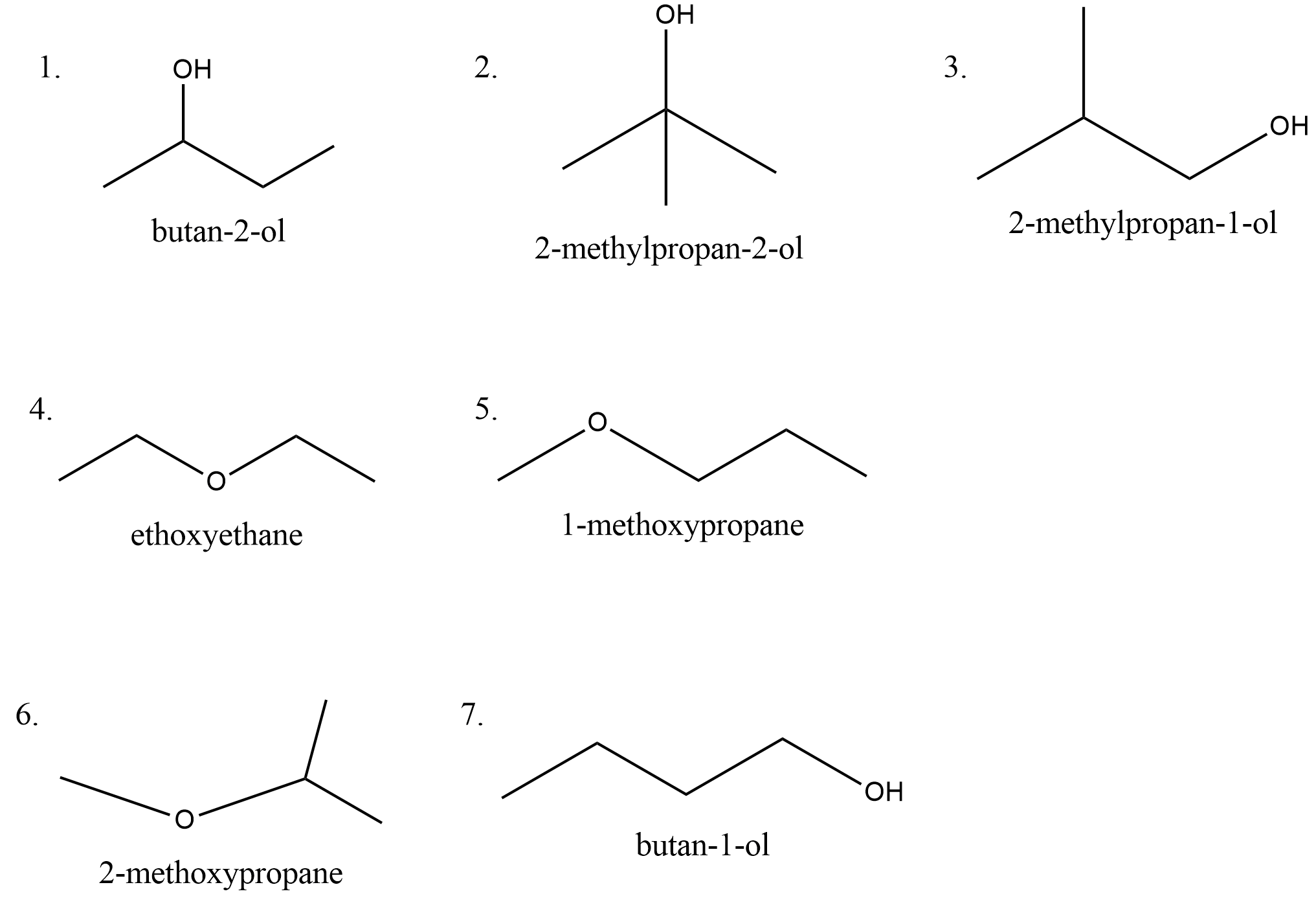
There are seven isomers possible which contain alcohols and ethers.
Hence the correct option is B.
Note:
Since the compound contains one oxygen atom, we may think that we can draw aldehyde and ketone as one of the isomers. But if we try to draw and aldehyde or ketone with $4$ carbons, only $8$ hydrogens are required. We have $10$ hydrogens in the molecular formula. Hence we don’t have an aldehyde or ketone as one of the isomers.
Complete step by step answer:
Given, vapour density of the compound is $37$. Molecular mass can be find out from vapour density using the formula,
Molecular mass = Vapour density $ \times 2$
Hence molecular mass of given compound is,
Molecular mass $ = 37 \times 2 = 74g/mol$
Given the organic compound contains $66\% $ carbon, $13.3\% $ hydrogen and the remaining is oxygen. Let x be the weight of carbon in the compound. Then we can write,
$
\dfrac{x}{{74}} \times 100 = 66 \\
x = \dfrac{{66 \times 74}}{{100}} = 48.84g \\
$
The compound contains $48.84g$ of carbon. Atomic mass of one carbon atom is $12g$. Hence number of carbons in the compound will be,
$\dfrac{{48.84}}{{12}} = 4.07$
We can round it to $4$ .
Similarly we can find out the number of hydrogens and oxygens present in the molecule.
Let y be the weight of hydrogens in the compound. Then we can write,
$
\dfrac{y}{{74}} \times 100 = 13.3 \\
y = \dfrac{{13.3 \times 74}}{{100}} = 9.842g \\
$
The compound contains $9.842g$ of hydrogen. Atomic mass of a hydrogen atom is $1g$. Hence number of hydrogens in the compound will be,
$\dfrac{{9.842}}{1} = 9.842$
We can round it to $10$ .
Let z be the weight of oxygen in the compound. The percentage of oxygen in the compound will be,
$20.7\% $ . Then we can write,
$
\dfrac{z}{{74}} \times 100 = 20.7 \\
z = \dfrac{{20.7 \times 74}}{{100}} = 15.318g \\
$
The compound contains $15.318g$ of oxygen. Atomic mass of an oxygen atom is $16g$. Hence number of oxygens in the compound will be,
$\dfrac{{15.318}}{{16}} = 0.9574$
We can round it to $1$ .
Hence the molecular formula of the compound is ${C_4}{H_{10}}O$ . Let us draw all the possible structures with this molecular formula. The possible structures are,

There are seven isomers possible which contain alcohols and ethers.
Hence the correct option is B.
Note:
Since the compound contains one oxygen atom, we may think that we can draw aldehyde and ketone as one of the isomers. But if we try to draw and aldehyde or ketone with $4$ carbons, only $8$ hydrogens are required. We have $10$ hydrogens in the molecular formula. Hence we don’t have an aldehyde or ketone as one of the isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




