
An organic acid (X) has the molecular formula ${{{C}}_{{8}}}{{{H}}_{{{10}}}}{{{O}}_{{2}}}$. (X) on heating with ${{N}}{{{H}}_{{3}}}$form (Y) which on treatment with alkaline ${{B}}{{{r}}_{{2}}}$forms. (Z) on treatment with ${{HN}}{{{O}}_{{2}}}$followed by heating with ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ gives ${{2,4 - dimethyl - 2 - pentane}}$. How many different acids (X) can give the indicated final product?
Answer
563.1k+ views
Hint: We draw the structure of eight carbon chains and such two oxygen functional groups which on reacting with ammonia gives amide as the next reaction is Hoffman bromamide reaction which acts on amide functional groups.
Complete step by step answer:
STEP ${{1}}$ Since the final product have ${{7}}$ carbon that means ${{1}}$ carbon with ${{2}}$oxygen can only form an ester or acid. Since we need only one carbon extra so it can be carboxylic acid only not ester as ester requires minimum ${{2}}$ carbon. In this step nucleophilic attack of ammonia happens to form amide.
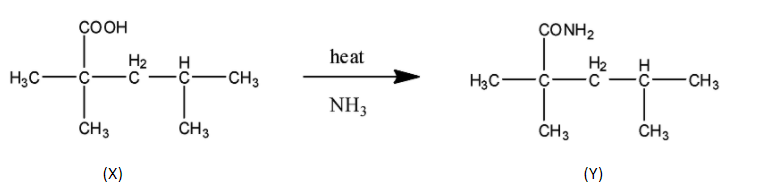
STEP ${{2}}$ Next step is the reaction with alkaline ${{B}}{{{r}}_{{2}}}$ which means ${{KOH/NaOH + B}}{{{r}}_{{2}}}$ which is a name reaction called Hoffmann bromamide. In this reaction the amide group is converted to the amine group.
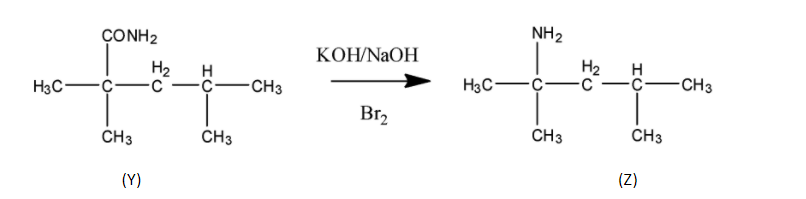
STEP ${{3}}$ In this step ${{HN}}{{{O}}_{{2}}}$ reacts with (Z) . This reaction is called diazotization in which the attacking nucleophile is ${{N}}{{{O}}^{{ + }}}$ which in presence of water form oxime and finally diazonium salt. So the product form in this step is a diazonium salt.
\[\]
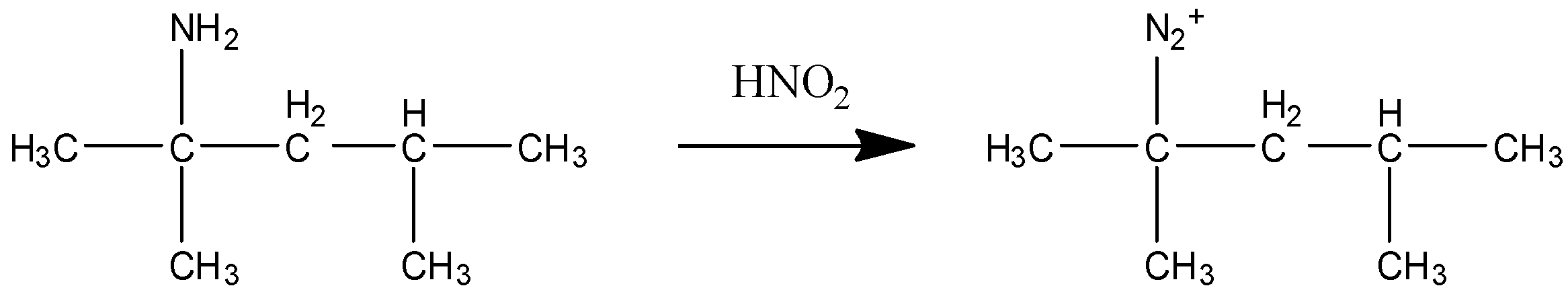
STEP 4 In this step ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ is in aqueous state where water ions are present. Since ${{{N}}_{{2}}}^{{ + }}$ is a strong leaving group the water present in the solution attacks there and is replaced as hydroxide. After alcohol is formed due to heating the elimination of alcohol group occurs and alkene is formed.
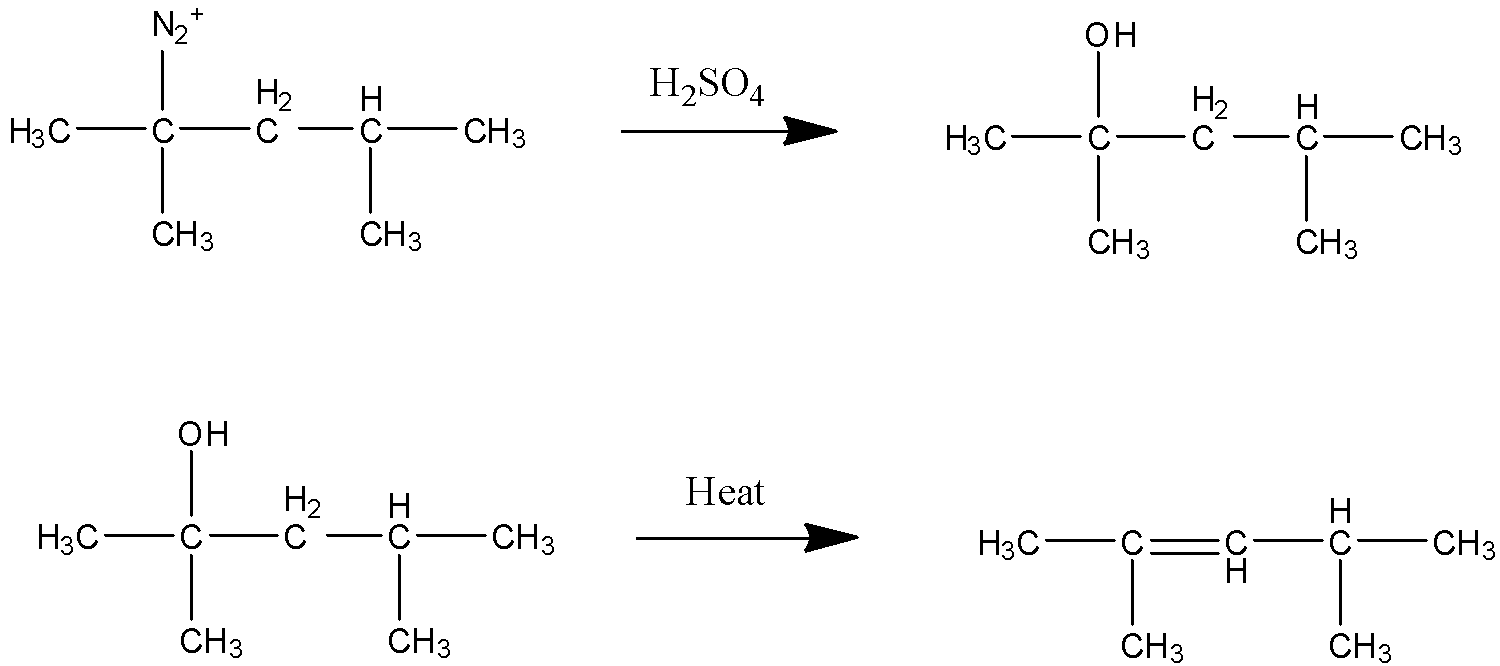
So the product (X) is given above
Additional information:
The diazotization reaction is a very important reaction. Since halo-benzenes are difficult to convert as they are a bad leaving group when attached directly to phenyl rings. So diazotization is the best reaction to convert those as good leaving groups and make different ranges of products.
Note: The carbonyl group of acid is electrophilic in nature and ammonia is basic so it attacks on the carbonyl group and when the lone pair reverses from the oxygen the ${{ - OH}}$ group leaves and amide is formed by addition on ${{ - N}}{{{H}}_{{2}}}$.
Complete step by step answer:
STEP ${{1}}$ Since the final product have ${{7}}$ carbon that means ${{1}}$ carbon with ${{2}}$oxygen can only form an ester or acid. Since we need only one carbon extra so it can be carboxylic acid only not ester as ester requires minimum ${{2}}$ carbon. In this step nucleophilic attack of ammonia happens to form amide.

STEP ${{2}}$ Next step is the reaction with alkaline ${{B}}{{{r}}_{{2}}}$ which means ${{KOH/NaOH + B}}{{{r}}_{{2}}}$ which is a name reaction called Hoffmann bromamide. In this reaction the amide group is converted to the amine group.

STEP ${{3}}$ In this step ${{HN}}{{{O}}_{{2}}}$ reacts with (Z) . This reaction is called diazotization in which the attacking nucleophile is ${{N}}{{{O}}^{{ + }}}$ which in presence of water form oxime and finally diazonium salt. So the product form in this step is a diazonium salt.
\[\]

STEP 4 In this step ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ is in aqueous state where water ions are present. Since ${{{N}}_{{2}}}^{{ + }}$ is a strong leaving group the water present in the solution attacks there and is replaced as hydroxide. After alcohol is formed due to heating the elimination of alcohol group occurs and alkene is formed.

So the product (X) is given above
Additional information:
The diazotization reaction is a very important reaction. Since halo-benzenes are difficult to convert as they are a bad leaving group when attached directly to phenyl rings. So diazotization is the best reaction to convert those as good leaving groups and make different ranges of products.
Note: The carbonyl group of acid is electrophilic in nature and ammonia is basic so it attacks on the carbonyl group and when the lone pair reverses from the oxygen the ${{ - OH}}$ group leaves and amide is formed by addition on ${{ - N}}{{{H}}_{{2}}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




