
An ideal gas is taken through a cycle \[A\to B\to C\to A\] as shown in figure. If the net heat supplied in the cycle is \[5J\], then work done by the gas in the process \[C\to A\] is
Answer
581.1k+ views
Hint: According to law of conservation of energy if I eat an apple then the apple will be converted into other forms of energy. The energy my body consumes from the apple can be converted into some work and also by changing my internal energy. This is an example of the first Law of Thermodynamics which is also true for conservation of energy. The energy we intake is equal to the difference of heat transfer to the system and work done by the system.
\[\Delta U=Q-W\]
\[\Delta U=\]change in Internal energy
Q = heat added by the system
If this heat is released then the system is exothermic and if absorbed then endothermic.
Formula used:
If a system is having the same initial and final position then the internal energy of the system is not changed.
\[\Delta U=\text{zero}\]
Complete answer:
We have been given that the system undergoes a cyclic process and returns to its initial state after a complete cycle. This is an cyclic process
\[A\to B\to C\to A\]
Hence \[\Delta U=0\text{ }\left( 1 \right)\]
According to first law of thermodynamics we have
\[Q=\Delta U+W\text{ }\left( 2 \right)\]
Net heat supplied to the system is \[5J\].
Using equation 1 and putting it in 2
\[\begin{align}
& W=5J \\
& {{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}=5J \\
\end{align}\]
Here \[{{W}_{AB}}=pressure\times change\text{ }in\text{ }volume\]
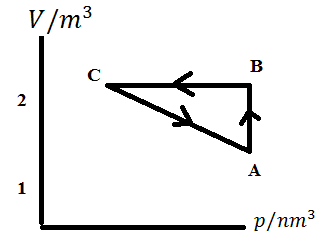
\[{{W}_{AB}}=10\times 1=10J\]
As we have no change in volume from \[B\to C\] Therefore \[{{W}_{BC}}=0\]
Hence \[{{W}_{CA}}+10J+0=5J\]
\[{{W}_{CA}}=-5J\]
Note:
First law of thermodynamics tells us about the conversion of energy from one form to another but it fails to explain in which direction the energy is flowing for direction purposes we use the 2nd Law of thermodynamics.
\[\Delta U=Q-W\]
\[\Delta U=\]change in Internal energy
Q = heat added by the system
If this heat is released then the system is exothermic and if absorbed then endothermic.
Formula used:
If a system is having the same initial and final position then the internal energy of the system is not changed.
\[\Delta U=\text{zero}\]
Complete answer:
We have been given that the system undergoes a cyclic process and returns to its initial state after a complete cycle. This is an cyclic process
\[A\to B\to C\to A\]
Hence \[\Delta U=0\text{ }\left( 1 \right)\]
According to first law of thermodynamics we have
\[Q=\Delta U+W\text{ }\left( 2 \right)\]
Net heat supplied to the system is \[5J\].
Using equation 1 and putting it in 2
\[\begin{align}
& W=5J \\
& {{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}=5J \\
\end{align}\]
Here \[{{W}_{AB}}=pressure\times change\text{ }in\text{ }volume\]

\[{{W}_{AB}}=10\times 1=10J\]
As we have no change in volume from \[B\to C\] Therefore \[{{W}_{BC}}=0\]
Hence \[{{W}_{CA}}+10J+0=5J\]
\[{{W}_{CA}}=-5J\]
Note:
First law of thermodynamics tells us about the conversion of energy from one form to another but it fails to explain in which direction the energy is flowing for direction purposes we use the 2nd Law of thermodynamics.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




