
An excited $H{e^ + }$ ion emits two photons in succession, with wavelengths 108.5 nm and 30.4 nm, in making a transition to ground state. The quantum number n, corresponding to its initial excited state is (for photon of wavelength $\lambda $, energy $E = \dfrac{{1240}}{{\lambda ({\text{in nm}})}}eV$)
A. n = 5
B. n = 4
C. n = 6
D. n = 7
Answer
575.4k+ views
Hint: The total energy emitted by the two de-excitations is equal to the sum of the energy of the two de-excitations and the Bohr model gives expression for total energy emitted when a hydrogen-like atom undergoes de-excitation from a higher energy state to lower energy state. By equating this formula and the sum of the given energies, we can find out the required value of n for the excited state.
Formula used:
According to Bohr model, the expression for total energy emitted when a hydrogen-like atom undergoes de-excitation from a higher energy state to lower energy state is given by the following formula.
$E = 13.6{Z^2}\left[ {\dfrac{1}{{n_1^2}} - \dfrac{1}{{n_2^2}}} \right]eV$
The energy is given in terms of the wavelength of a light by the following expression.
$E = \dfrac{{hc}}{\lambda }$
Complete step by step answer:
We are given an excited \[H{e^ + }\] ion which is a hydrogen-like atom. It emits two photons in succession while making a transition to the ground state from the excited state. The two wavelengths emitted are given as
$
{\lambda _1} = 108.5nm \\
{\lambda _2} = 30.4nm \\
$
The following diagram shows the energy levels for the given atom where the de-excitation takes from an energy level n to the ground state n = 1
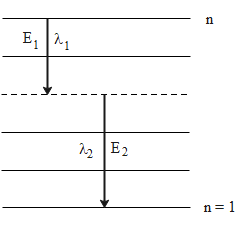
The energy emitted by a hydrogen-like atom in a single de-excitation from an excited state ${n_2}$ to a lower energy state ${n_1}$ is given by the following formula.
$E = 13.6{Z^2}\left[ {\dfrac{1}{{n_1^2}} - \dfrac{1}{{n_2^2}}} \right]eV$
Now for the given atom, Z = 2 and the total energy emitted by the two de-excitations is equal to the sum of the energy of the two de-excitations. Therefore, we have
$
E = {E_1} + {E_2} \\
\Rightarrow 13.6{Z^2}\left[ {\dfrac{1}{{{1^2}}} - \dfrac{1}{{{n^2}}}} \right] = \dfrac{{hc}}{{{\lambda _1}}} + \dfrac{{hc}}{{{\lambda _2}}} \\
\Rightarrow 13.6 \times {\left( 2 \right)^2}\left[ {\dfrac{1}{{{1^2}}} - \dfrac{1}{{{n^2}}}} \right] = \dfrac{{1240eV/nm}}{{108.5nm}} + \dfrac{{1240eV/nm}}{{30.4nm}} = 11.43 + 40.79 = 52.22 \\
\Rightarrow 1 - \dfrac{1}{{{n^2}}} = \dfrac{{52.22}}{{54.4}} \\
\Rightarrow \dfrac{1}{{{n^2}}} = 1 - \dfrac{{52.22}}{{54.4}} = \dfrac{{2.18}}{{54.4}} \\
\Rightarrow {n^2} = \dfrac{{54.4}}{{2.18}} = 25 \\
\therefore n = 5 \\
$
This is the required value of n for the excited state. Hence, the de-excitation takes from n = 5 state, i.e. the fourth excited state,
So, the correct answer is option A.
Note:
Hydrogen-like atoms are those which have the same electronic configuration as that of a neutral hydrogen atom. The neutral helium atom is not hydrogen-like but if we remove one electron from it then we get the same electronic configuration as hydrogen. Similarly, other hydrogen-like atoms are $L{i^{2 + }}$, $B{e^{3 + }}$, etc.
Formula used:
According to Bohr model, the expression for total energy emitted when a hydrogen-like atom undergoes de-excitation from a higher energy state to lower energy state is given by the following formula.
$E = 13.6{Z^2}\left[ {\dfrac{1}{{n_1^2}} - \dfrac{1}{{n_2^2}}} \right]eV$
The energy is given in terms of the wavelength of a light by the following expression.
$E = \dfrac{{hc}}{\lambda }$
Complete step by step answer:
We are given an excited \[H{e^ + }\] ion which is a hydrogen-like atom. It emits two photons in succession while making a transition to the ground state from the excited state. The two wavelengths emitted are given as
$
{\lambda _1} = 108.5nm \\
{\lambda _2} = 30.4nm \\
$
The following diagram shows the energy levels for the given atom where the de-excitation takes from an energy level n to the ground state n = 1

The energy emitted by a hydrogen-like atom in a single de-excitation from an excited state ${n_2}$ to a lower energy state ${n_1}$ is given by the following formula.
$E = 13.6{Z^2}\left[ {\dfrac{1}{{n_1^2}} - \dfrac{1}{{n_2^2}}} \right]eV$
Now for the given atom, Z = 2 and the total energy emitted by the two de-excitations is equal to the sum of the energy of the two de-excitations. Therefore, we have
$
E = {E_1} + {E_2} \\
\Rightarrow 13.6{Z^2}\left[ {\dfrac{1}{{{1^2}}} - \dfrac{1}{{{n^2}}}} \right] = \dfrac{{hc}}{{{\lambda _1}}} + \dfrac{{hc}}{{{\lambda _2}}} \\
\Rightarrow 13.6 \times {\left( 2 \right)^2}\left[ {\dfrac{1}{{{1^2}}} - \dfrac{1}{{{n^2}}}} \right] = \dfrac{{1240eV/nm}}{{108.5nm}} + \dfrac{{1240eV/nm}}{{30.4nm}} = 11.43 + 40.79 = 52.22 \\
\Rightarrow 1 - \dfrac{1}{{{n^2}}} = \dfrac{{52.22}}{{54.4}} \\
\Rightarrow \dfrac{1}{{{n^2}}} = 1 - \dfrac{{52.22}}{{54.4}} = \dfrac{{2.18}}{{54.4}} \\
\Rightarrow {n^2} = \dfrac{{54.4}}{{2.18}} = 25 \\
\therefore n = 5 \\
$
This is the required value of n for the excited state. Hence, the de-excitation takes from n = 5 state, i.e. the fourth excited state,
So, the correct answer is option A.
Note:
Hydrogen-like atoms are those which have the same electronic configuration as that of a neutral hydrogen atom. The neutral helium atom is not hydrogen-like but if we remove one electron from it then we get the same electronic configuration as hydrogen. Similarly, other hydrogen-like atoms are $L{i^{2 + }}$, $B{e^{3 + }}$, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




