
An ester of molecular formula \[{{C}_{4}}{{H}_{8}}{{O}_{2}}\] was produced by the reaction of an alcohol with a carboxylic acid.
Alcohol Acid 1 Methanol Propanoic acid 2 Ethanol Ethanoic acid 3 Propanol Propanoic acid
Which of the following could be the alcohol and the acid?
a.2
b.1 and 2
c.1 and 3
d.1, 2 and 3
| Alcohol | Acid | |
| 1 | Methanol | Propanoic acid |
| 2 | Ethanol | Ethanoic acid |
| 3 | Propanol | Propanoic acid |
Answer
598.8k+ views
Hint: The given question states that the reactants are acid and carboxylic acid and the product is an ester. Start this question by drawing out the possible structures of ester with the molecular formula \[{{C}_{4}}{{H}_{8}}{{O}_{2}}\].
Complete step by step answer:
As we can see, the process occurring here is esterification, which is the reaction between alcohols and carboxylic acids to make esters. The general reaction is as follows –
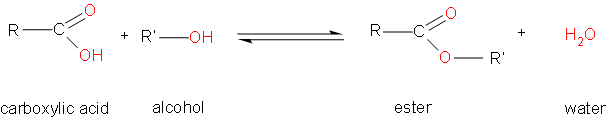
According to the question, the compound has 4 carbons.
Therefore, the reaction can either be between methanol and propionic acid or ethanol or ethanoic acid to give \[{{C}_{4}}{{H}_{8}}{{O}_{2}}\].
Therefore, the answer is – option (b).
Additional Information: Other than this, esters are also formed by a reaction between acyl chlorides (acid chlorides) and alcohols, and between acid anhydrides and alcohols.
Note: Let us define esters, alcohols and carboxylic acids –
“Esters have a hydrocarbon group of some sort replacing the hydrogen in the -COOH group of a carboxylic acid. We shall just be looking at cases where it is replaced by an alkyl group, but it could equally well be an aryl group (one based on a benzene ring).”
“Carboxylic acids are compounds which contain a -COOH group. For the purposes of this page we shall just look at compounds where the -COOH group is attached either to a hydrogen atom or to an alkyl group.”
“Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group.”
Complete step by step answer:
As we can see, the process occurring here is esterification, which is the reaction between alcohols and carboxylic acids to make esters. The general reaction is as follows –

According to the question, the compound has 4 carbons.
Therefore, the reaction can either be between methanol and propionic acid or ethanol or ethanoic acid to give \[{{C}_{4}}{{H}_{8}}{{O}_{2}}\].
Therefore, the answer is – option (b).
Additional Information: Other than this, esters are also formed by a reaction between acyl chlorides (acid chlorides) and alcohols, and between acid anhydrides and alcohols.
Note: Let us define esters, alcohols and carboxylic acids –
“Esters have a hydrocarbon group of some sort replacing the hydrogen in the -COOH group of a carboxylic acid. We shall just be looking at cases where it is replaced by an alkyl group, but it could equally well be an aryl group (one based on a benzene ring).”
“Carboxylic acids are compounds which contain a -COOH group. For the purposes of this page we shall just look at compounds where the -COOH group is attached either to a hydrogen atom or to an alkyl group.”
“Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group.”
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




