
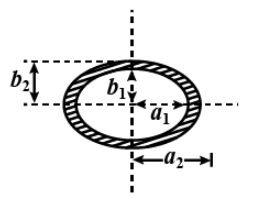
An elliptically shaped ring of dimensions shown in figure just touches the horizontal surface of a liquid of surface tension $S$. The force required to pull the ring away from the liquid surface is
(A) $2\pi \left( {\sqrt {{a_1}{b_1}} + \sqrt {{a_2}{b_2}} } \right)S$
(B) $\pi \left( {{a_1} + {b_1} + {a_2} + {b_2}} \right)S$
(C) $\pi \left( {\dfrac{{{a_1} + {a_2}}}{2} + \dfrac{{{b_1} + {b_2}}}{2}} \right)S$
(D) $\sqrt 2 \pi \left( {\sqrt {{a_1}{b_1}} + \sqrt {{a_2}{b_2}} } \right)S$

Answer
581.1k+ views
Hint:Here first we find the internal mean radius and also the circumference of the ring. Then we again find the external mean radius and external circumference. Then we can get the force that is required by using the formula of surface tension.
Complete step by step answer:
Given,
An elliptically shaped ring of dimensions shown in figure just touches the horizontal surface of a liquid of surface tension $S$.
The mean radius of the inner ring is
${r_1} = \sqrt {{a_1}{b_1}} $
Now, the circumference of the inner ring is
$2\pi {r_1} = 2\pi \left( {\sqrt {{a_1}{b_1}} } \right)$
The mean radius of the external ring is
${r_2} = \sqrt {{a_2}{b_2}} $
The circumference of the outer ring is
$2\pi {r_2} = 2\pi \left( {\sqrt {{a_2}{b_2}} } \right)$
To find the force we have to add the circumferences of the ring and multiply with surface tension $S$.
So, the required force$ = 2\pi \left( {\sqrt {{a_1}{b_1}} + \sqrt {{a_2}{b_2}} } \right)S$
Hence, option A is the correct answer.
Additional information:
Surface tension: The attractive force exerted by the molecules below on the surface molecules of a liquid appears to pull the surface molecules into the bulk of the liquid and makes the liquid adopt the form that has the least surface area.The ratio of the surface force $F$ to the length $L$ along which the force exists is known as surface tension. Mathematically, surface tension is written as-
$S = F/L$
The results of intermolecular forces at the interface create surface tension. Surface tension depends on the composition of the material, temperature and the ambient environment. Liquids were molecules that would have a high surface tension and strong attractive intermolecular forces.
Note:Here while calculating the radius we have to see that we are calculating the mean radius. So, we have to put the total radius under square root.
Complete step by step answer:
Given,
An elliptically shaped ring of dimensions shown in figure just touches the horizontal surface of a liquid of surface tension $S$.
The mean radius of the inner ring is
${r_1} = \sqrt {{a_1}{b_1}} $
Now, the circumference of the inner ring is
$2\pi {r_1} = 2\pi \left( {\sqrt {{a_1}{b_1}} } \right)$
The mean radius of the external ring is
${r_2} = \sqrt {{a_2}{b_2}} $
The circumference of the outer ring is
$2\pi {r_2} = 2\pi \left( {\sqrt {{a_2}{b_2}} } \right)$
To find the force we have to add the circumferences of the ring and multiply with surface tension $S$.
So, the required force$ = 2\pi \left( {\sqrt {{a_1}{b_1}} + \sqrt {{a_2}{b_2}} } \right)S$
Hence, option A is the correct answer.
Additional information:
Surface tension: The attractive force exerted by the molecules below on the surface molecules of a liquid appears to pull the surface molecules into the bulk of the liquid and makes the liquid adopt the form that has the least surface area.The ratio of the surface force $F$ to the length $L$ along which the force exists is known as surface tension. Mathematically, surface tension is written as-
$S = F/L$
The results of intermolecular forces at the interface create surface tension. Surface tension depends on the composition of the material, temperature and the ambient environment. Liquids were molecules that would have a high surface tension and strong attractive intermolecular forces.
Note:Here while calculating the radius we have to see that we are calculating the mean radius. So, we have to put the total radius under square root.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




