
An electrochemical cell generally consists of?
A. A cathode, anode, electrolyte, wire and two compartments
B. Cathode, anode, and wire
C. Two compartment that conduct electricity
D. A positive and negative side
E. A cathode and an anode
Answer
572.1k+ views
Hint: An electrochemical cell is a device which converts chemical energy into electrical energy. It does so by passing electrons, occurring during chemical reactions, through wire attached to electrodes. So, these electrodes are termed as anode and cathode.
Complete step by step answer:
In order to understand this, let us draw a diagram consisting of anode and cathode.
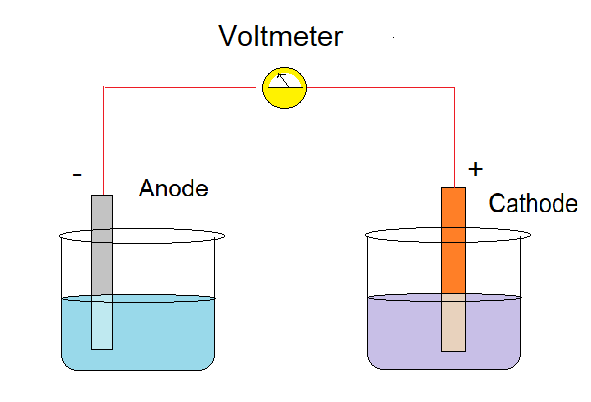
As we can see in the figure the electrochemical cell just consists of anode and cathode.
Wire and electrolytes are external and extra parts which are used to consume cell energy.
Cathode is a place where reduction occurs and anode is a place of oxidation.
Oxidation means loss of electrons while reduction means gain of electrons. That's why electrons get transferred from anode to cathode because anode oxidation takes place.
Cathode is denoted by positive sign as electron enters in it whereas, anode as negative sign as electron moves out of it.
So, the correct answer is Option E.
Additional information :
Electrochemical cells are used to convert chemical energy into electrical energy by some chemical reaction which provides the electric energy. It is also used to carry chemical reactions by supplying electrical energy. Generally there are two types of electrochemical cells. These are galvanic and electrolytic cells.
Note: Generally while making the electrochemical cell diagram it is convention to make the cathode on the right hand side and the anode at left side. Electrochemical cells play a very important and crucial role in our life. Some advanced electrochemical cells like fuel cells serve the very purpose of clean energy in remote locations.
Complete step by step answer:
In order to understand this, let us draw a diagram consisting of anode and cathode.

As we can see in the figure the electrochemical cell just consists of anode and cathode.
Wire and electrolytes are external and extra parts which are used to consume cell energy.
Cathode is a place where reduction occurs and anode is a place of oxidation.
Oxidation means loss of electrons while reduction means gain of electrons. That's why electrons get transferred from anode to cathode because anode oxidation takes place.
Cathode is denoted by positive sign as electron enters in it whereas, anode as negative sign as electron moves out of it.
So, the correct answer is Option E.
Additional information :
Electrochemical cells are used to convert chemical energy into electrical energy by some chemical reaction which provides the electric energy. It is also used to carry chemical reactions by supplying electrical energy. Generally there are two types of electrochemical cells. These are galvanic and electrolytic cells.
Note: Generally while making the electrochemical cell diagram it is convention to make the cathode on the right hand side and the anode at left side. Electrochemical cells play a very important and crucial role in our life. Some advanced electrochemical cells like fuel cells serve the very purpose of clean energy in remote locations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




