
An atom that has electronic configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$ , you will place in which of the following group:
A. Fifth
B. Fifteen
C. Second
D. Third
Answer
567k+ views
Hint: Electronic configuration is defined as the way in which the electrons are distributed in its atomic orbitals. It follows a standard notation where all the electrons containing subshells are placed in a sequence.
Complete step by step answer:
Electronic configuration is defined as the way in which the electrons are distributed in its atomic orbitals.
There is a standard notation which it follows where all the electrons containing subshells are placed in a sequence.
The standard notation of electronic configuration is given below:
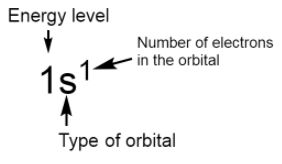
General electronic configurations of different blocks are given below:
1.Alkali metals: $n{s^1}$
2.Alkali earth metals: $n{s^2}$
3.P block elements: $n{s^2}n{p^{1 - 6}}$
4.D-block elements: $\left( {n - 1} \right){d^{1 - 10}}n{s^{1 - 2}}$
5.F block elements: $\left( {n - 2} \right){f^{0 - 14}}\left( {n - 1} \right){d^{0 - 1}}n{s^2}$
To check the group of the atom which it belongs to through the electronic configuration then we have to add the electrons from the outermost shell and the shell prior to it.
The outermost shell and the one shell prior to it that is d orbital consists of total $5$ electrons which means that the atom belongs to the d block.
d- block starts from group $3$ to group $12$ .
So, option b) fifteen and option c) second will be eradicated because group $15$ comes under the p block and group $2$ comes under the s block.
Now, as we know there are a total of $5$ electrons in their outermost shell and the shell prior to it, so the above atom belongs to the group $5$ of d block elements.
So, the correct answer is Option A.
Note: We will never get to know the exact group of an element just by the electrons that are present in their outermost shell. The electrons in the outermost shell will only let you know in which block it
Complete step by step answer:
Electronic configuration is defined as the way in which the electrons are distributed in its atomic orbitals.
There is a standard notation which it follows where all the electrons containing subshells are placed in a sequence.
The standard notation of electronic configuration is given below:

General electronic configurations of different blocks are given below:
1.Alkali metals: $n{s^1}$
2.Alkali earth metals: $n{s^2}$
3.P block elements: $n{s^2}n{p^{1 - 6}}$
4.D-block elements: $\left( {n - 1} \right){d^{1 - 10}}n{s^{1 - 2}}$
5.F block elements: $\left( {n - 2} \right){f^{0 - 14}}\left( {n - 1} \right){d^{0 - 1}}n{s^2}$
To check the group of the atom which it belongs to through the electronic configuration then we have to add the electrons from the outermost shell and the shell prior to it.
The outermost shell and the one shell prior to it that is d orbital consists of total $5$ electrons which means that the atom belongs to the d block.
d- block starts from group $3$ to group $12$ .
So, option b) fifteen and option c) second will be eradicated because group $15$ comes under the p block and group $2$ comes under the s block.
Now, as we know there are a total of $5$ electrons in their outermost shell and the shell prior to it, so the above atom belongs to the group $5$ of d block elements.
So, the correct answer is Option A.
Note: We will never get to know the exact group of an element just by the electrons that are present in their outermost shell. The electrons in the outermost shell will only let you know in which block it
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




