
An athlete takes 100gm of glucose of energy equivalent to 1560 KJ. How much energy is taken up by 1gm molecule of glucose?
[A] 15.6 KJ
[B] 2808 KJ
[C] 1560 KJ
[D] 28.08 KJ
Answer
578.4k+ views
Hint: To solve this, firstly you have to remember that the chemical formula of glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. Find out molecular weight of glucose from the molecular formula. Remember that molar mass of any substance is the number of grams of the substance in its molecular form.
Complete answer:
Before answering this, let us discuss what glucose is.
Glucose is basically a sugar which is a carbohydrate. It is a monosaccharide with the chemical formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. It has 5 hydroxyl groups which are arranged in a specific way along the six carbon parent chains. We can draw its structure as-
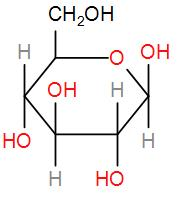
Now, in the given question, we have that 100 grams of glucose has energy equivalent to 1560 KJ.
We know that chemical formula of glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$ therefore, its molecular weight will be 180 g/mol.
Now, 100 g of glucose gives 1560 KJ energy.
We know that 1 mole of glucose will be equivalent to the number of atoms of glucose present in 1 molecule of glucose.
So, 1 mole of glucose = molecular weight of glucose = 180 g
So, for 1 g molecule of glucose, we will have $\dfrac{1560}{100}\times 180\text{ KJ=2808 KJ}$
So, amount of energy up taken by 1gm of glucose = 2808 KJ
Therefore, the correct answer is option [B] 2808 KJ.
Note:
We should remember that glucose acts as an energy source. When we intake glucose, it carries energy that can be transported by cells of our body.
During metabolism, when glucose is oxidised, it produces carbon dioxide and other nitrogen based compounds. This process provides energy which can be used up by the cells.
Our main source of energy is carbohydrates and glucose as we have already discussed above is a carbohydrate too.
Complete answer:
Before answering this, let us discuss what glucose is.
Glucose is basically a sugar which is a carbohydrate. It is a monosaccharide with the chemical formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. It has 5 hydroxyl groups which are arranged in a specific way along the six carbon parent chains. We can draw its structure as-

Now, in the given question, we have that 100 grams of glucose has energy equivalent to 1560 KJ.
We know that chemical formula of glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$ therefore, its molecular weight will be 180 g/mol.
Now, 100 g of glucose gives 1560 KJ energy.
We know that 1 mole of glucose will be equivalent to the number of atoms of glucose present in 1 molecule of glucose.
So, 1 mole of glucose = molecular weight of glucose = 180 g
So, for 1 g molecule of glucose, we will have $\dfrac{1560}{100}\times 180\text{ KJ=2808 KJ}$
So, amount of energy up taken by 1gm of glucose = 2808 KJ
Therefore, the correct answer is option [B] 2808 KJ.
Note:
We should remember that glucose acts as an energy source. When we intake glucose, it carries energy that can be transported by cells of our body.
During metabolism, when glucose is oxidised, it produces carbon dioxide and other nitrogen based compounds. This process provides energy which can be used up by the cells.
Our main source of energy is carbohydrates and glucose as we have already discussed above is a carbohydrate too.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




