
An aromatic compound A of the molecular formula ${{C}_{8}}{{H}_{10}}O$ on reaction with iodine and dilute NaOH gives a yellow precipitate. The structure of the compound is expected to be:
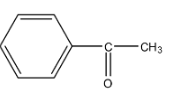
A.
B. ${{C}_{6}}{{H}_{5}}-CHOH-C{{H}_{3}}$
C.
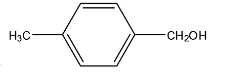
D.
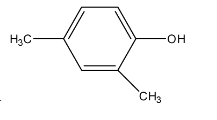



Answer
534.3k+ views
Hint: The compound has molecular formula ${{C}_{8}}{{H}_{10}}O$, since it has one oxygen, then the compound will be alcohol, an ester, or a ketone. The second information given is that the compound forms a yellow precipitate with iodine and NaOH, this means that the compound gives the Iodoform test.
Complete step by step solution: The compound has molecular formula ${{C}_{8}}{{H}_{10}}O$, since it has one oxygen, then the compound will be alcohol, an ester, or a ketone. The second information given is that the compound forms a yellow precipitate with iodine and NaOH, this means that the compound gives the Iodoform test.
So, the compounds that will positive test for the Iodoform test when it give a yellow precipitate with iodine and dilute NaOH, and there is a condition for the compound to give the Iodoform test is the presence of $-COC{{H}_{3}}$ group.
So, in the given options there are two compounds in which the $-COC{{H}_{3}}$ group is present, i.e., option (a) and option (b). The molecular formula of the compound in option (a) is ${{C}_{8}}{{H}_{8}}O$ and the molecular formula of the compound in option (b) is ${{C}_{8}}{{H}_{10}}O$.
So, the reaction is given below:
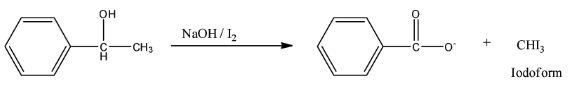
So, this compound is secondary alcohol and gives yellow precipitate in the Iodoform test.
Therefore, the correct answer is an option (b).
Note: Iodoform is an important test that is used to distinguish between methyl ketone and non-methyl ketones. A compound that doesn't give the Iodoform test is formaldehyde or methanal.
Complete step by step solution: The compound has molecular formula ${{C}_{8}}{{H}_{10}}O$, since it has one oxygen, then the compound will be alcohol, an ester, or a ketone. The second information given is that the compound forms a yellow precipitate with iodine and NaOH, this means that the compound gives the Iodoform test.
So, the compounds that will positive test for the Iodoform test when it give a yellow precipitate with iodine and dilute NaOH, and there is a condition for the compound to give the Iodoform test is the presence of $-COC{{H}_{3}}$ group.
So, in the given options there are two compounds in which the $-COC{{H}_{3}}$ group is present, i.e., option (a) and option (b). The molecular formula of the compound in option (a) is ${{C}_{8}}{{H}_{8}}O$ and the molecular formula of the compound in option (b) is ${{C}_{8}}{{H}_{10}}O$.
So, the reaction is given below:

So, this compound is secondary alcohol and gives yellow precipitate in the Iodoform test.
Therefore, the correct answer is an option (b).
Note: Iodoform is an important test that is used to distinguish between methyl ketone and non-methyl ketones. A compound that doesn't give the Iodoform test is formaldehyde or methanal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




