
An aromatic compound ‘A’ having molecular formula ${C_7}{H_6}{O_2}$ on treated with aqueous ammonia heating forms compound ‘B’. The compound ‘B’ in reaction with molecular bromine and potassium hydroxide provides compound ‘C’; having molecular formula ${C_6}{H_7}N$ . The structure of ‘A’ is:
A.
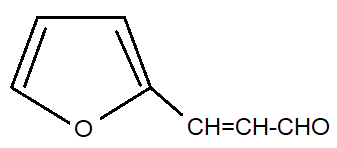
B.
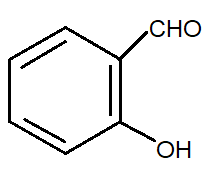
C.
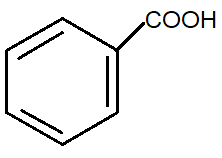



Answer
547.2k+ views
Hint: In order to determine the structure of compound ‘A’ , firstly we will find the degree of unsaturation in every given compound. And also we know that bromine molecule and sodium hydroxide is Hoffmann Bromamide reagent which gives an idea that compound ‘B’ is an amide.
Complete step by step answer:
According to the question,
The molecular formula of compound ‘A’ is ${C_7}{H_6}{O_2}$ :
So, degree of unsaturation of compound ‘A’ is : $5$
Now, the molecular formula of compound ‘C’ is ${C_6}{H_7}N$ :
So, the degree of unsaturation of compound ‘C’ is: $4$
Now as per the question when ${C_7}{H_6}{O_2}$ reacts with aqueous ammonia and heated then ${C_7}{H_7}ON$ forms that is compound ‘B’ and the reaction takes place as:
\[\;{C_6}{H_6}{O_2} + aq.N{H_3}\xrightarrow{\Delta }{C_7}{H_7}ON\]
So, the degree of unsaturation of compound ‘B’ is: $5$
The reagent used in the reaction $B{r_2}/NaOH$ is classic Hoffmann Bromamide reagent. And also according to the question compound ‘B’ is treated with Hoffmann bromamide reagent that means compound ‘B’ is an amide. As it consumes one degree of unsaturation. Compound ‘B’ has six carbon atoms and five hydrogen atoms then we can conclude that compound ‘B’ is benzamide.
Now, benzamide on Hofmann degradation gives aniline that is the formula of compound ‘C’. The reaction takes place as:
${C_7}{H_7}ON + B{r_2}/NaOH \to {C_6}{H_7}N$
Generally, ammonia and heat on heating with carboxylic acid gives amide. This means compound ‘A’ is a carboxylic acid. So among all the options, option C is benzoic acid.
Hence, option C is correct.
Note:
When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, degradation of amide takes place leading to the formation of primary amine. This reaction involves degradation of amide and is popularly known as Hoffmann bromamide degradation reaction.
Complete step by step answer:
According to the question,
The molecular formula of compound ‘A’ is ${C_7}{H_6}{O_2}$ :
So, degree of unsaturation of compound ‘A’ is : $5$
Now, the molecular formula of compound ‘C’ is ${C_6}{H_7}N$ :
So, the degree of unsaturation of compound ‘C’ is: $4$
Now as per the question when ${C_7}{H_6}{O_2}$ reacts with aqueous ammonia and heated then ${C_7}{H_7}ON$ forms that is compound ‘B’ and the reaction takes place as:
\[\;{C_6}{H_6}{O_2} + aq.N{H_3}\xrightarrow{\Delta }{C_7}{H_7}ON\]
So, the degree of unsaturation of compound ‘B’ is: $5$
The reagent used in the reaction $B{r_2}/NaOH$ is classic Hoffmann Bromamide reagent. And also according to the question compound ‘B’ is treated with Hoffmann bromamide reagent that means compound ‘B’ is an amide. As it consumes one degree of unsaturation. Compound ‘B’ has six carbon atoms and five hydrogen atoms then we can conclude that compound ‘B’ is benzamide.
Now, benzamide on Hofmann degradation gives aniline that is the formula of compound ‘C’. The reaction takes place as:
${C_7}{H_7}ON + B{r_2}/NaOH \to {C_6}{H_7}N$
Generally, ammonia and heat on heating with carboxylic acid gives amide. This means compound ‘A’ is a carboxylic acid. So among all the options, option C is benzoic acid.
Hence, option C is correct.
Note:
When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, degradation of amide takes place leading to the formation of primary amine. This reaction involves degradation of amide and is popularly known as Hoffmann bromamide degradation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




