
An amine ${{\text{C}}_3}{{\text{H}}_9}{\text{N}}$ reacts with benzene sulfonyl chloride to form a white precipitate which is insoluble in aqueous ${\text{NaOH}}$. The amine is:
A.
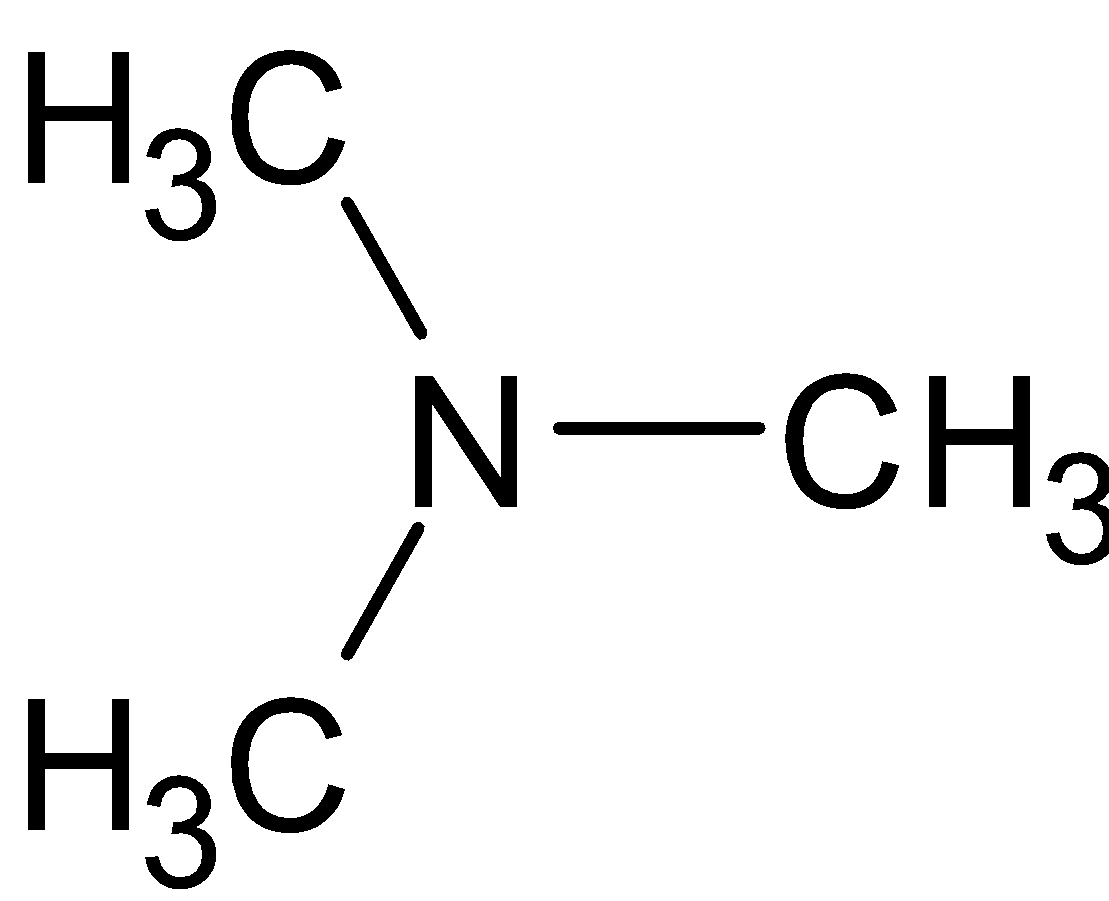
B.
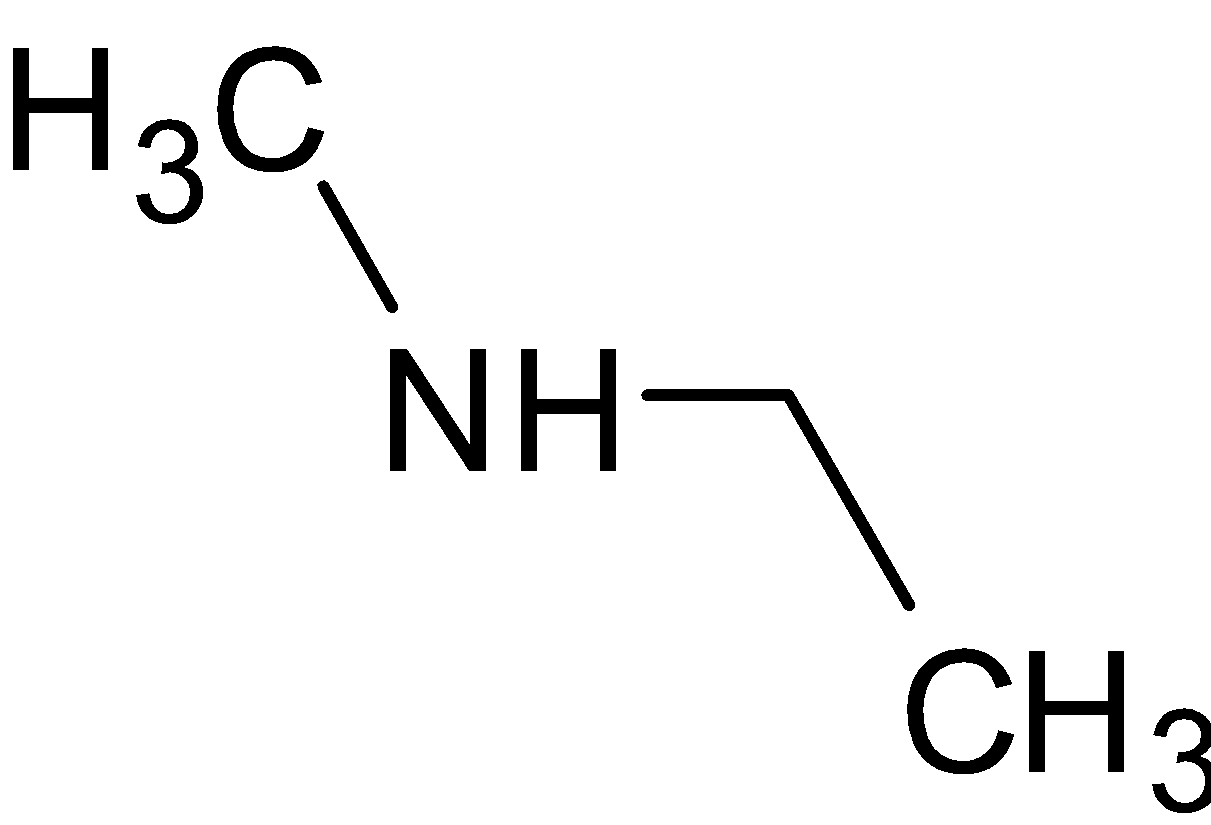
C.
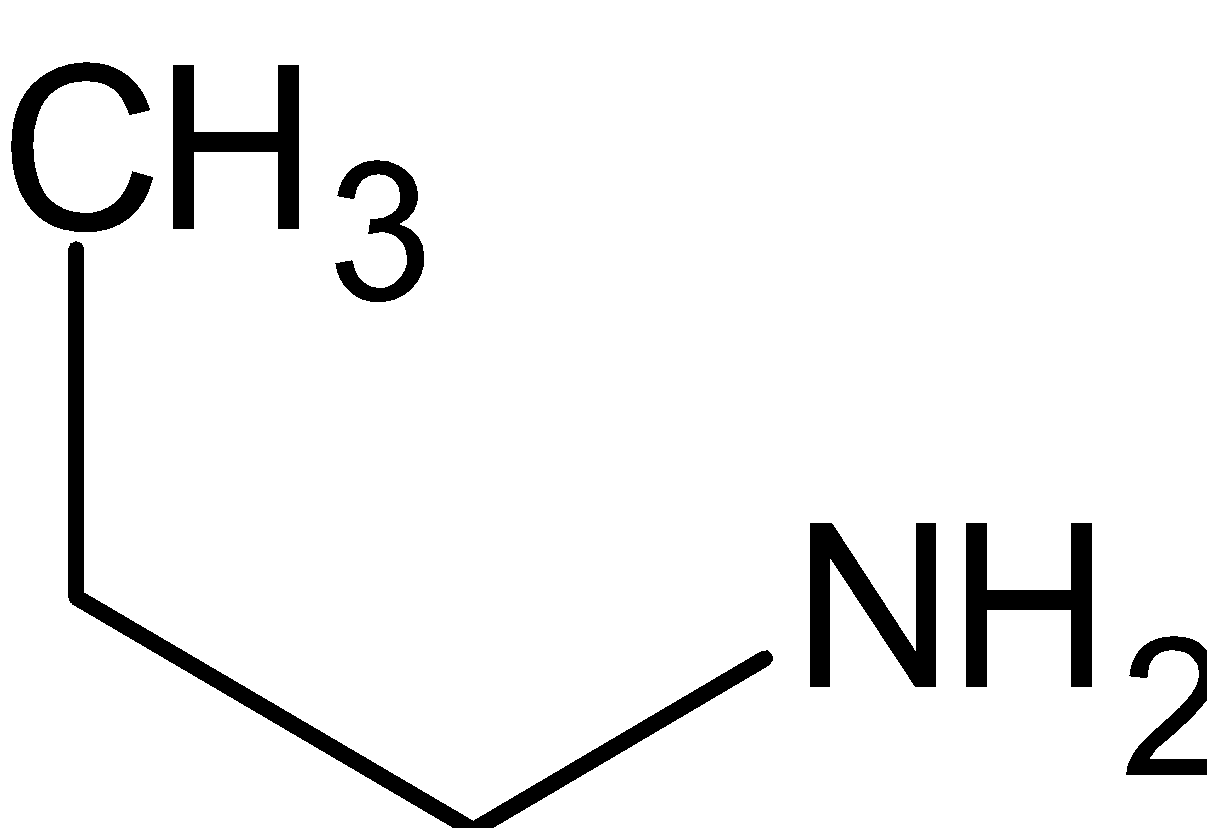
D.
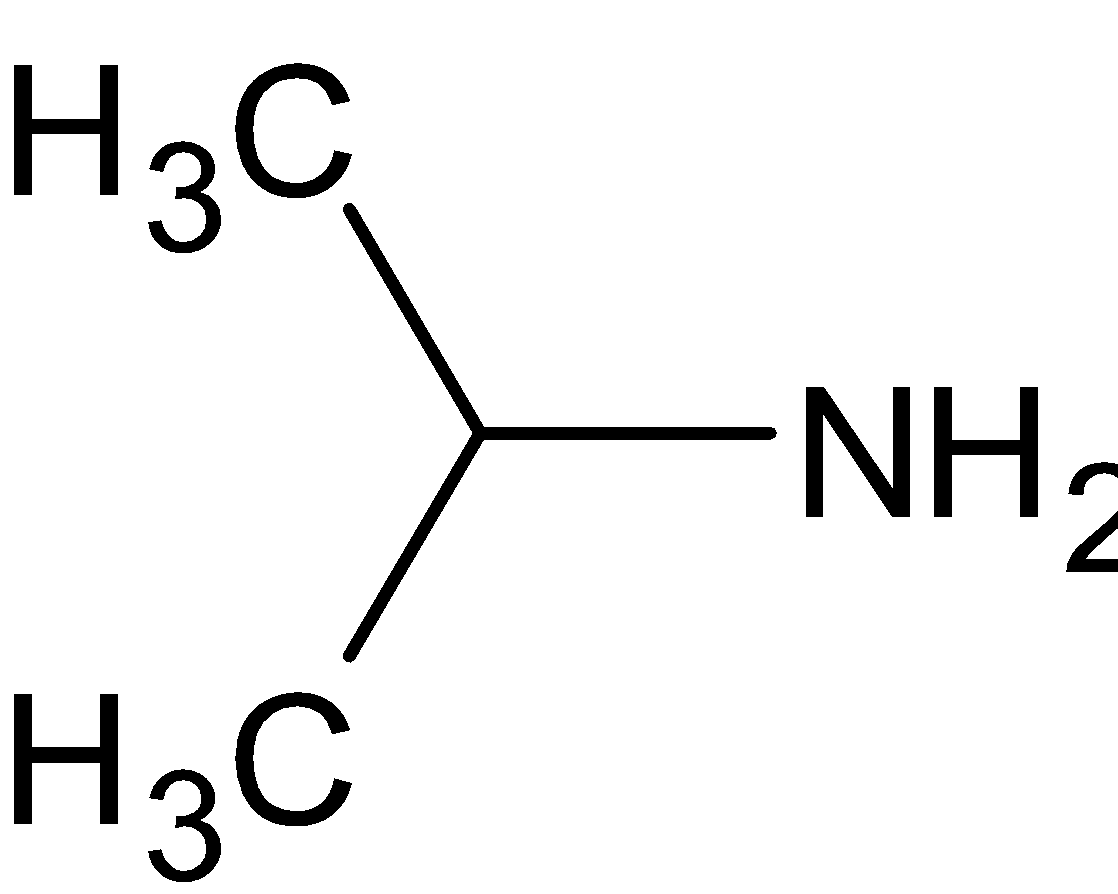




Answer
589.5k+ views
Hint: Primary, secondary, and tertiary amines are traced and discerned using Hinsberg test. Benzene sulfonyl chloride is known as the Hinsberg reagent. It does not react with tertiary amines properly. Along with the Hinsberg reagent, aqueous ${\text{KOH}}$ is also added. So based on the solubility of the product formed, three of them can be differentiated.
Given data:
Given that a nitrogen containing compound reacts with Hinsberg reagent which gives an alkali insoluble compound.
Complete step by step answer:
Amines are organic compounds having one or more nitrogen groups. Amines are divided into primary, secondary, tertiary, and aromatic amines. Primary, secondary, tertiary amines are detected and distinguished by the Hinsberg test.
Benzene sulfonyl chloride is represented as ${\text{PhS}}{{\text{O}}_2}{\text{Cl}}$. Benzene sulfonic acid or its salt is chlorinated with phosphorus oxychloride which produces benzene sulfonyl chloride.
${\text{PhS}}{{\text{O}}_3}^ - {\text{N}}{{\text{a}}^ + }\xrightarrow[{{\text{POC}}{{\text{l}}_3}}]{{{\text{PC}}{{\text{l}}_5}}}{\text{PhS}}{{\text{O}}_2}{\text{Cl}}$
Benzene sulfonyl chloride.
A.
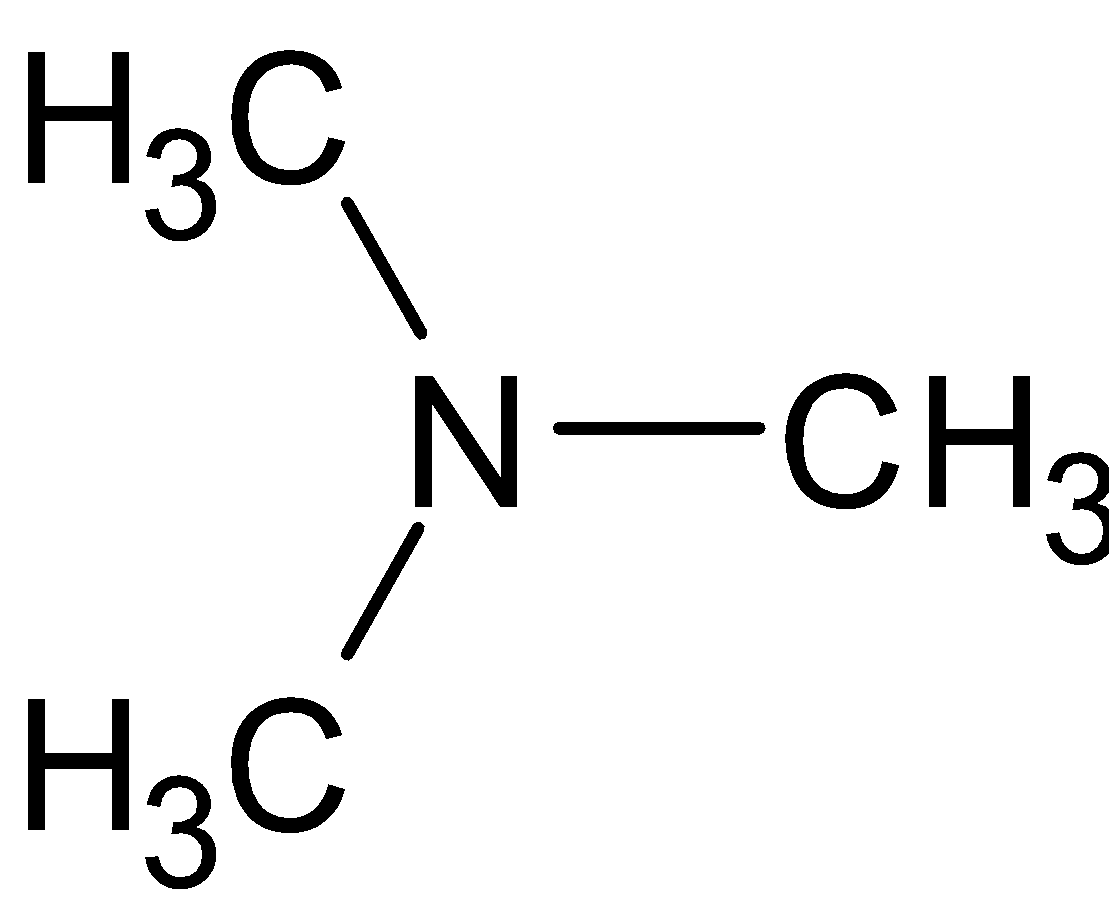 is an example of tertiary amines since they have three alkyl groups directly attached to nitrogen. . Tertiary amines can react with Hinsberg reagent under certain conditions only. Reaction speed, concentration, solubility and temperature have to be considered.
is an example of tertiary amines since they have three alkyl groups directly attached to nitrogen. . Tertiary amines can react with Hinsberg reagent under certain conditions only. Reaction speed, concentration, solubility and temperature have to be considered.
${\text{PhS}}{{\text{O}}_2}{\text{Cl}} + {{\text{R}}_3}{\text{N}} + {{\text{H}}_2}{\text{O}} \to {{\text{R}}_3}{\text{N}}{{\text{H}}^ + }\left[ {{\text{PhS}}{{\text{O}}_3}^ - } \right] + {\text{HCl}}$
This gives water-soluble sulfonate salts.
B.
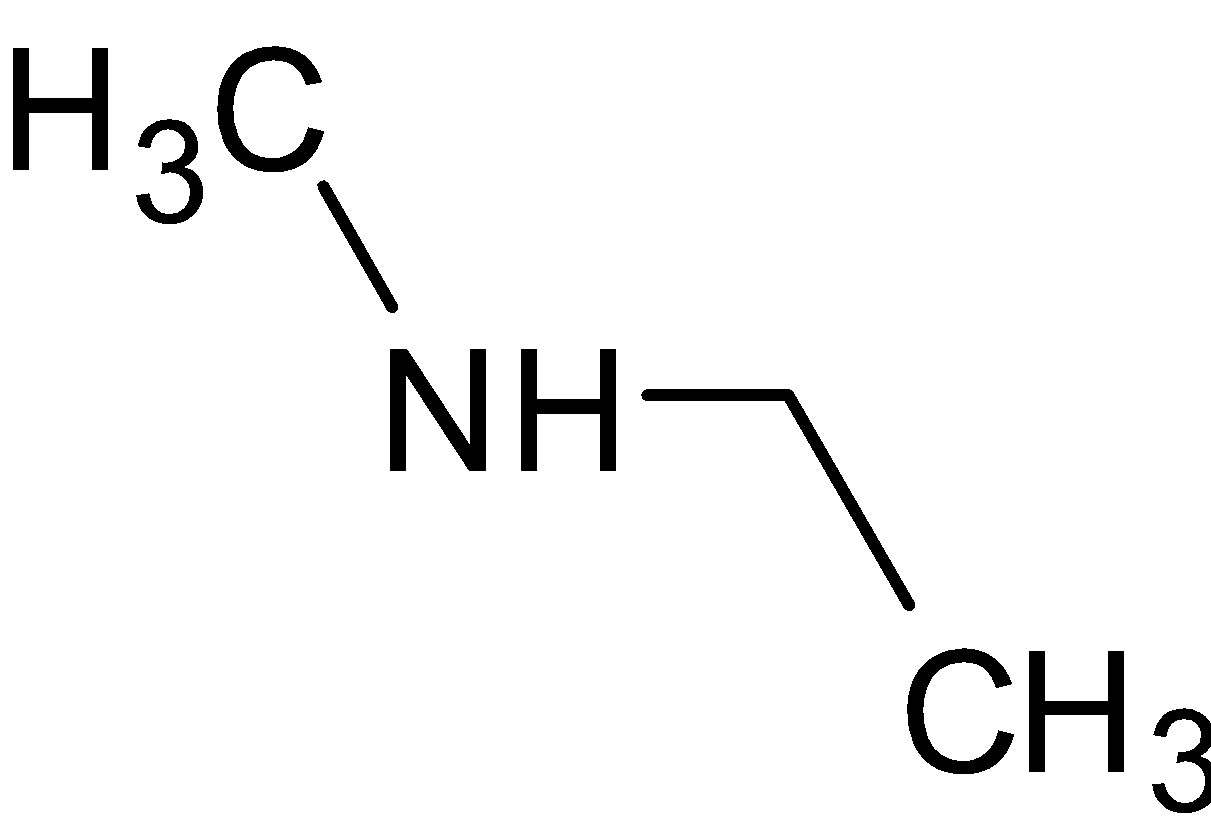 is a secondary amine since it has two alkyl groups attached to nitrogen.
is a secondary amine since it has two alkyl groups attached to nitrogen.
There is a direct formation of an alkali insoluble sulfonamide when secondary amine is treated with Hinsberg reagent.
\[{\text{PhS}}{{\text{O}}_2}{\text{Cl}} + 2{\text{RR}}'{\text{NH}} \to {\text{PhS}}{{\text{O}}_2}{\text{NRR' + HCl}}\]
Benzene sulfonyl chloride
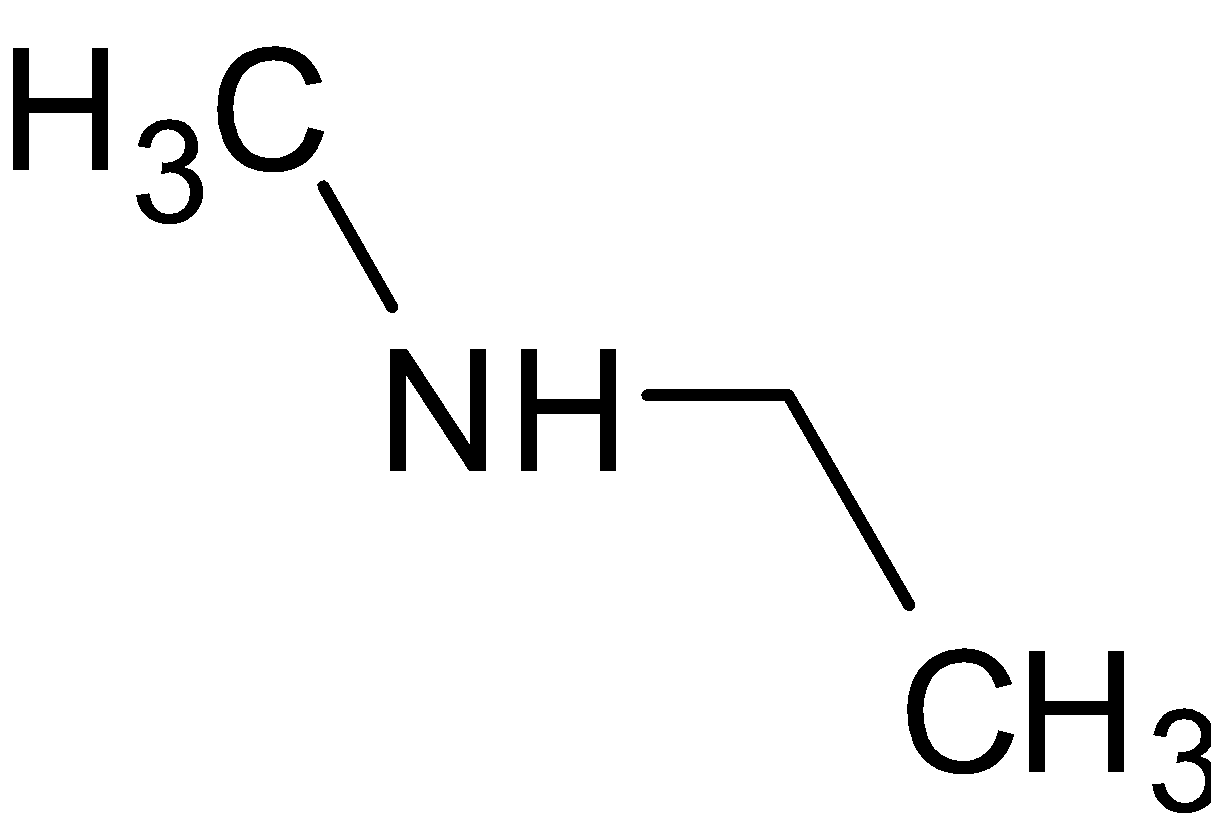 $ + {\text{PhS}}{{\text{O}}_2}{\text{Cl}} \to $${\text{PhS}}{{\text{O}}_2}{\text{ - N}}\left( {{\text{C}}{{\text{H}}_3}} \right)\left( {{{\text{C}}_2}{{\text{H}}_5}} \right)$$ + {\text{HCl}}$
$ + {\text{PhS}}{{\text{O}}_2}{\text{Cl}} \to $${\text{PhS}}{{\text{O}}_2}{\text{ - N}}\left( {{\text{C}}{{\text{H}}_3}} \right)\left( {{{\text{C}}_2}{{\text{H}}_5}} \right)$$ + {\text{HCl}}$
Benzene sulfonyl chloride
C.
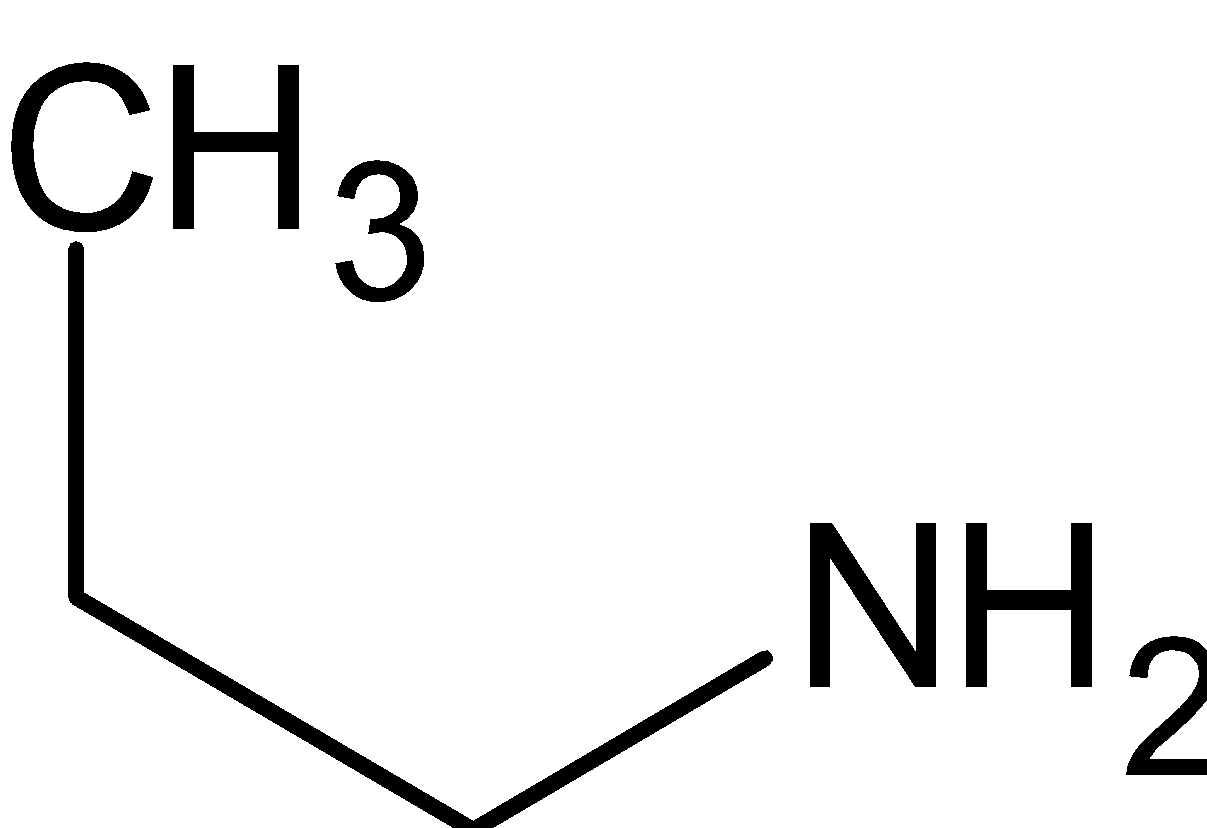 is a primary amine since it has only one alkyl group directly attached to nitrogen.
is a primary amine since it has only one alkyl group directly attached to nitrogen.
When primary amine is reacted with Hinsberg reagent, it forms a sulfonamide which on reaction with base gives sulfonamide salt. It is water-soluble.
\[{\text{PhS}}{{\text{O}}_2}{\text{N}}\left( {\text{H}} \right){\text{R}} + {\text{NaOH}} \to {\text{N}}{{\text{a}}^ + }\left[ {{\text{PhS}}{{\text{O}}_2}{\text{N}}{{\text{R}}^ - }} \right] + {{\text{H}}_2}{\text{O}}\]
Sulfonamide Sulfonamide salt
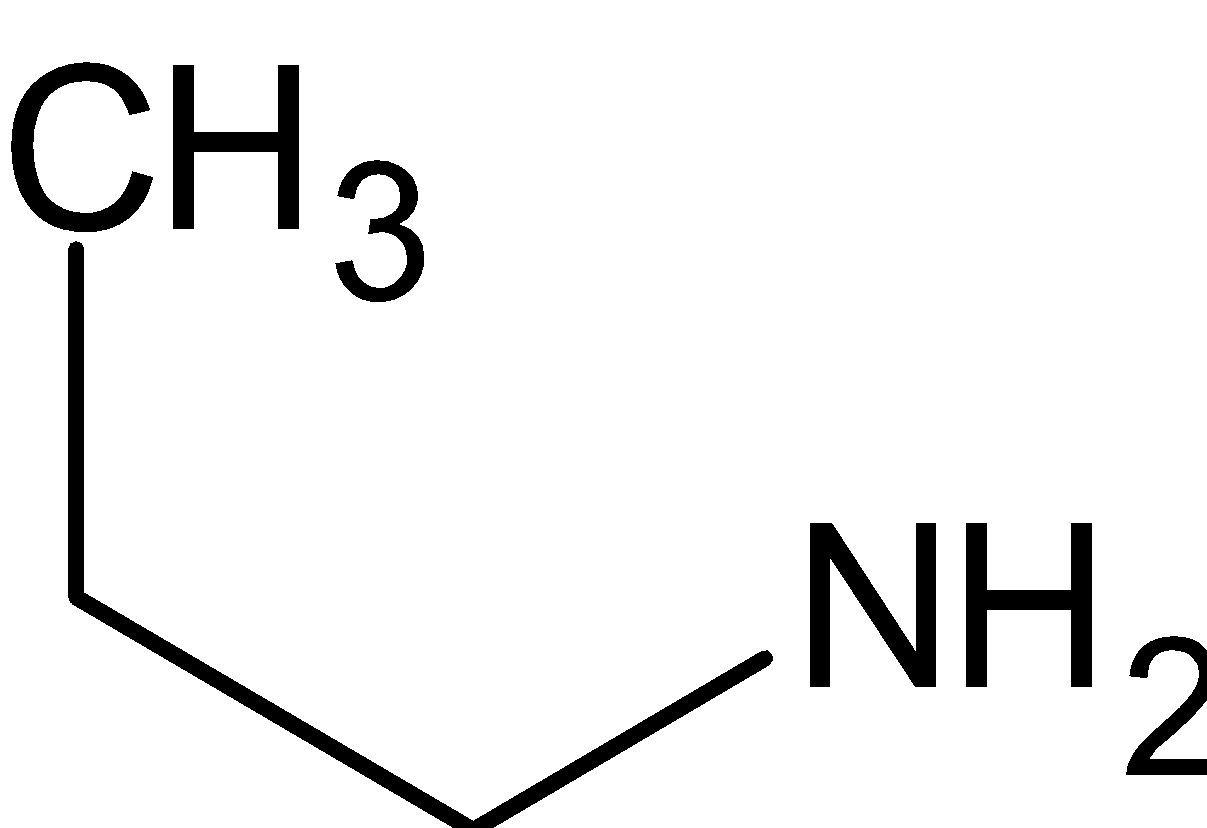 $ + {\text{PhS}}{{\text{O}}_2}{\text{Cl}} \to {\text{PhS}}{{\text{O}}_2}{\text{NH}}\left( {{{\text{C}}_2}{{\text{H}}_5}} \right) + {\text{HCl}}$
$ + {\text{PhS}}{{\text{O}}_2}{\text{Cl}} \to {\text{PhS}}{{\text{O}}_2}{\text{NH}}\left( {{{\text{C}}_2}{{\text{H}}_5}} \right) + {\text{HCl}}$
D.
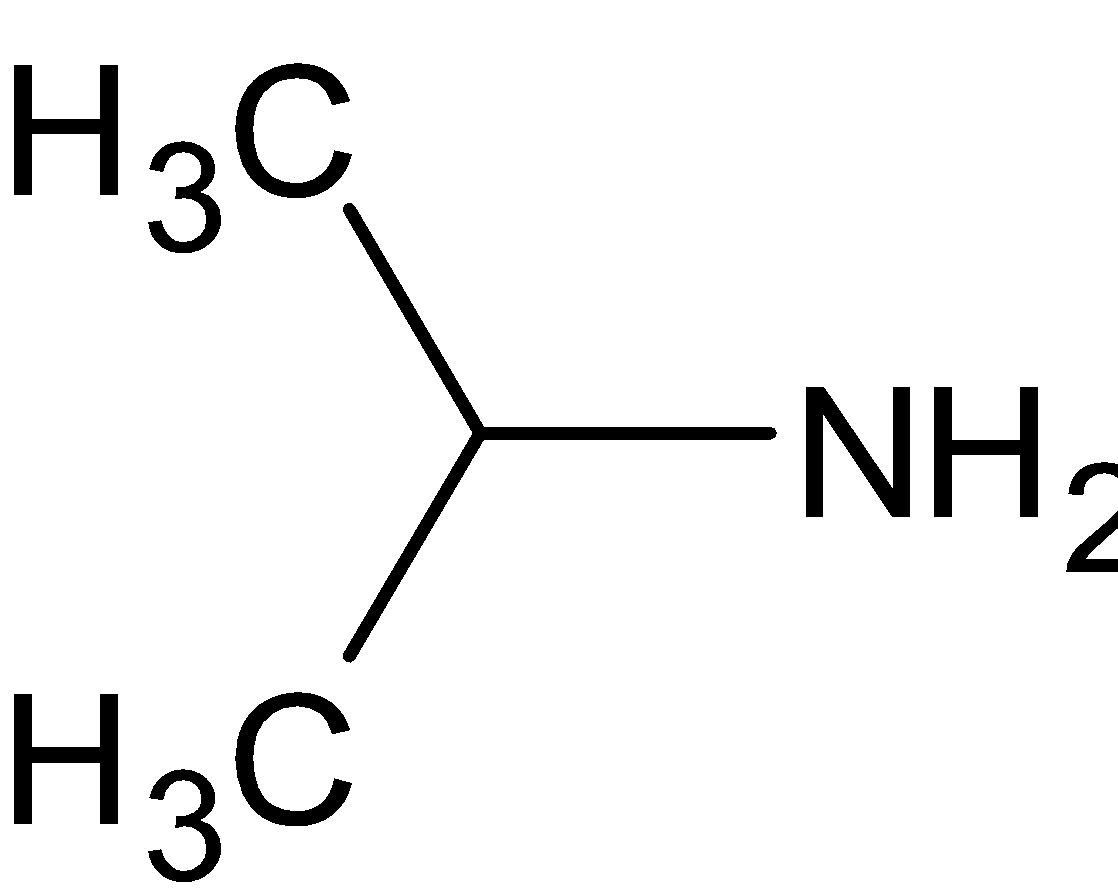 is a primary amine since it has only one alkyl group directly attached. It is similar to the above option.
is a primary amine since it has only one alkyl group directly attached. It is similar to the above option.
Hence the option B is correct.
Additional information:
Here the nucleophile is amine and the electrophile is sulfonyl chloride. Nucleophile amine is attacking the electrophile sulfonyl chloride. In the case of primary amines, when treated with Hinsberg reagent, the alkali helps to lose the proton and forms a water soluble sulfonamide salt.
Note:
Sulfonyl chloride undergoes hydrolysis when reacted with tertiary amines which produces water soluble sulfonate salts. Differentiation of primary, secondary, tertiary amines are based on solubility of the product in alkali.
Given data:
Given that a nitrogen containing compound reacts with Hinsberg reagent which gives an alkali insoluble compound.
Complete step by step answer:
Amines are organic compounds having one or more nitrogen groups. Amines are divided into primary, secondary, tertiary, and aromatic amines. Primary, secondary, tertiary amines are detected and distinguished by the Hinsberg test.
Benzene sulfonyl chloride is represented as ${\text{PhS}}{{\text{O}}_2}{\text{Cl}}$. Benzene sulfonic acid or its salt is chlorinated with phosphorus oxychloride which produces benzene sulfonyl chloride.
${\text{PhS}}{{\text{O}}_3}^ - {\text{N}}{{\text{a}}^ + }\xrightarrow[{{\text{POC}}{{\text{l}}_3}}]{{{\text{PC}}{{\text{l}}_5}}}{\text{PhS}}{{\text{O}}_2}{\text{Cl}}$
Benzene sulfonyl chloride.
A.

${\text{PhS}}{{\text{O}}_2}{\text{Cl}} + {{\text{R}}_3}{\text{N}} + {{\text{H}}_2}{\text{O}} \to {{\text{R}}_3}{\text{N}}{{\text{H}}^ + }\left[ {{\text{PhS}}{{\text{O}}_3}^ - } \right] + {\text{HCl}}$
This gives water-soluble sulfonate salts.
B.

There is a direct formation of an alkali insoluble sulfonamide when secondary amine is treated with Hinsberg reagent.
\[{\text{PhS}}{{\text{O}}_2}{\text{Cl}} + 2{\text{RR}}'{\text{NH}} \to {\text{PhS}}{{\text{O}}_2}{\text{NRR' + HCl}}\]
Benzene sulfonyl chloride

Benzene sulfonyl chloride
C.

When primary amine is reacted with Hinsberg reagent, it forms a sulfonamide which on reaction with base gives sulfonamide salt. It is water-soluble.
\[{\text{PhS}}{{\text{O}}_2}{\text{N}}\left( {\text{H}} \right){\text{R}} + {\text{NaOH}} \to {\text{N}}{{\text{a}}^ + }\left[ {{\text{PhS}}{{\text{O}}_2}{\text{N}}{{\text{R}}^ - }} \right] + {{\text{H}}_2}{\text{O}}\]
Sulfonamide Sulfonamide salt

D.

Hence the option B is correct.
Additional information:
Here the nucleophile is amine and the electrophile is sulfonyl chloride. Nucleophile amine is attacking the electrophile sulfonyl chloride. In the case of primary amines, when treated with Hinsberg reagent, the alkali helps to lose the proton and forms a water soluble sulfonamide salt.
Note:
Sulfonyl chloride undergoes hydrolysis when reacted with tertiary amines which produces water soluble sulfonate salts. Differentiation of primary, secondary, tertiary amines are based on solubility of the product in alkali.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




