
An amide with the formula ${{C}_{2}}{{H}_{5}}NO$
Answer
582.6k+ views
Hint:Try to recall that by calculating the DBE of a given compound we can easily give the formula of its amide and also, the DBE of amide is 1. Now, by using this you can easily give the formula of amide.
Complete answer:
It is known to you that DBE stands for Double Bond Equivalent is also known as the level of unsaturation. DBE is the number of unsaturation present in an organic molecule.The term unsaturated means a double bond or a ring system.It is the number of hydrogen molecules that would have to be added to single bonds and all rings to acyclic structures.
The DBE number can be calculated from the formula using the following equation:
$\Rightarrow DBE=\dfrac{2n-m+p+2}{2}$
where, n= number of carbon atoms in organic molecules, m=number of hydrogen atoms and p= number of nitrogen atoms. If oxygen and sulphur is there then neglect them in calculation of DBE. Also, treat halogens like hydrogen in calculation of DBE of an organic molecule.
Calculation of DBE for given formula:
Given, n=2, m=5 and p=1
$DBE=\dfrac{2n-m+p+2}{2}$
$\Rightarrow \text{ }\dfrac{(2\text{ x 2) - 5 + 1 + 2}}{2}\text{ = 1}$
Since, DBE is one, so there will be a double bond between carbon and oxygen for amide.
Hence, an amide with formula ${{C}_{2}}{{H}_{5}}NO$ is
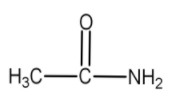
Note:It should be remembered to you that if for any organic molecule DBE is greater than four, then it should have at least one benzene ring in its structure.
Also, you should remember that if an organic molecule has DBE=1 and has two oxygen atoms, then it must have a carboxylic acid group.
Complete answer:
It is known to you that DBE stands for Double Bond Equivalent is also known as the level of unsaturation. DBE is the number of unsaturation present in an organic molecule.The term unsaturated means a double bond or a ring system.It is the number of hydrogen molecules that would have to be added to single bonds and all rings to acyclic structures.
The DBE number can be calculated from the formula using the following equation:
$\Rightarrow DBE=\dfrac{2n-m+p+2}{2}$
where, n= number of carbon atoms in organic molecules, m=number of hydrogen atoms and p= number of nitrogen atoms. If oxygen and sulphur is there then neglect them in calculation of DBE. Also, treat halogens like hydrogen in calculation of DBE of an organic molecule.
Calculation of DBE for given formula:
Given, n=2, m=5 and p=1
$DBE=\dfrac{2n-m+p+2}{2}$
$\Rightarrow \text{ }\dfrac{(2\text{ x 2) - 5 + 1 + 2}}{2}\text{ = 1}$
Since, DBE is one, so there will be a double bond between carbon and oxygen for amide.
Hence, an amide with formula ${{C}_{2}}{{H}_{5}}NO$ is

Note:It should be remembered to you that if for any organic molecule DBE is greater than four, then it should have at least one benzene ring in its structure.
Also, you should remember that if an organic molecule has DBE=1 and has two oxygen atoms, then it must have a carboxylic acid group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




