
An alcohol used as an antifreeze compound is ethylene glycol.
A. True
B. False
Answer
585.3k+ views
Hint: Alcohol is an organic compound in which the hydroxyl group is bound with a saturated carbon atom. Primary alcohol(ethanol) is used as a drug. Based on the number of \[ - OH\] groups there are two kinds of alcohol, one is gem diol and the other one is a vicinal diol.
Complete step by step answer:
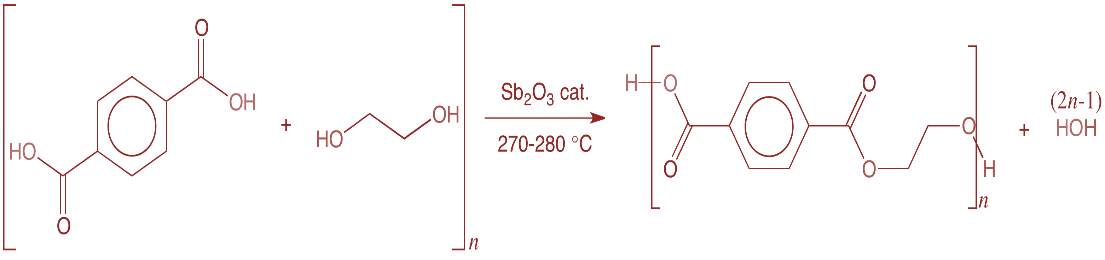
Ethylene glycol is a vicinal diol. The IUPAC name is ethylene 1,2 diol. It is used as a Raw material to synthesis polyester fibers. For example, terylene fiber is a synthetic polymer. It is produced by the condensation reaction mechanism between ethylene glycol and terephthalic acid. The reaction is shown below,
Among alcohols, this compound can be used as the anti-frozen compound. It can decrease the freezing point of the substance. The structure of ethylene glycol is,
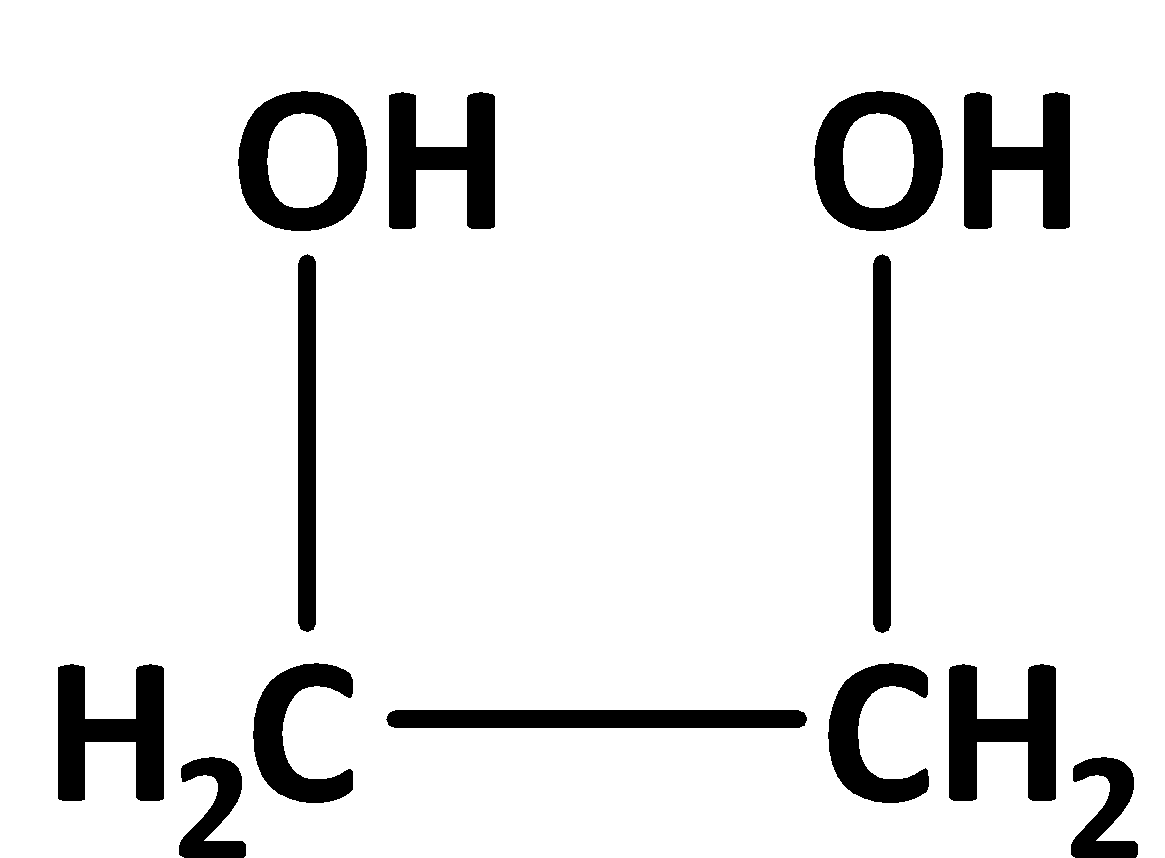
So, the given statement is true.
The correct option is A.
Additional information:
When the two \[ - OH\] groups are attached at the same carbon of the organic compound then it is called gem diol. And when the \[ - OH\] groups are in two different but neighboring carbon then it is called vicinal diol.
The gem diols are less stable than vicinal diols
The stability of gem diols can be increased in the presence of the electron-withdrawing group at the alpha position. +I effect decreases the stability of the gem diol and increases the extent of dehydration as follows.
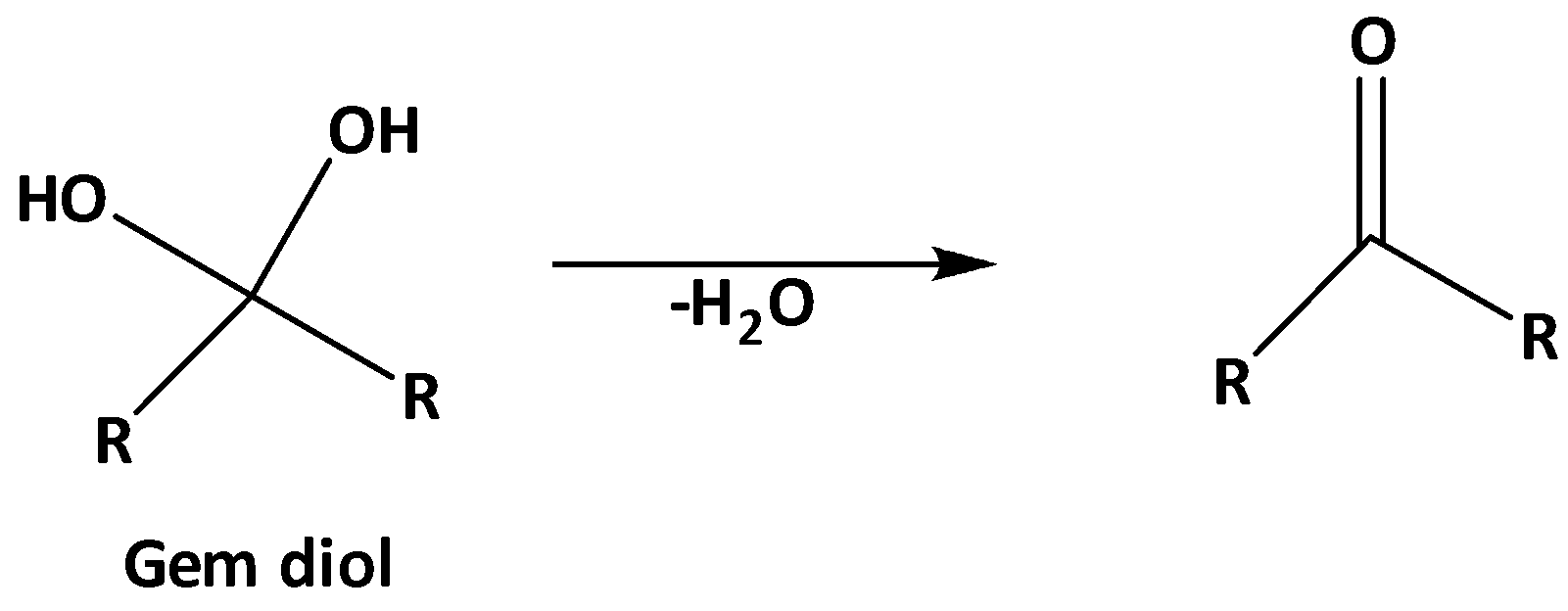
And -I effect increases the stability of gem diol. As well as the intramolecular hydrogen bonding also increases the stability of gem diol.
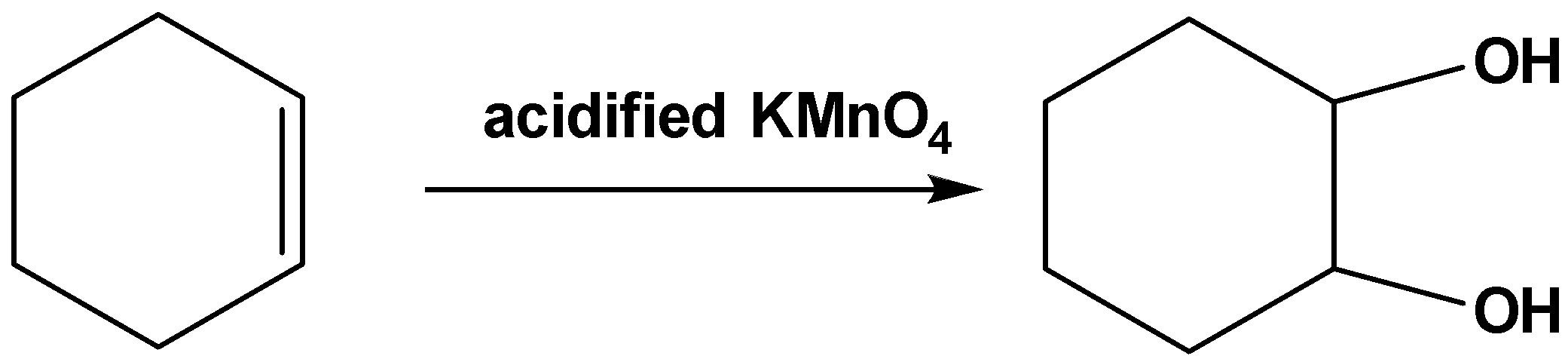
Note:The vicinal diols can be formed by oxidation of the double bonds. For example, when acidified \[\;KMn{O_4}\] reacts with cyclohexene oxidation of the double bonds takes place and vicinal diol is formed as follows,
Complete step by step answer:
Ethylene glycol is a vicinal diol. The IUPAC name is ethylene 1,2 diol. It is used as a Raw material to synthesis polyester fibers. For example, terylene fiber is a synthetic polymer. It is produced by the condensation reaction mechanism between ethylene glycol and terephthalic acid. The reaction is shown below,

Among alcohols, this compound can be used as the anti-frozen compound. It can decrease the freezing point of the substance. The structure of ethylene glycol is,

So, the given statement is true.
The correct option is A.
Additional information:
When the two \[ - OH\] groups are attached at the same carbon of the organic compound then it is called gem diol. And when the \[ - OH\] groups are in two different but neighboring carbon then it is called vicinal diol.
The gem diols are less stable than vicinal diols
The stability of gem diols can be increased in the presence of the electron-withdrawing group at the alpha position. +I effect decreases the stability of the gem diol and increases the extent of dehydration as follows.

And -I effect increases the stability of gem diol. As well as the intramolecular hydrogen bonding also increases the stability of gem diol.
Note:The vicinal diols can be formed by oxidation of the double bonds. For example, when acidified \[\;KMn{O_4}\] reacts with cyclohexene oxidation of the double bonds takes place and vicinal diol is formed as follows,

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




