
Amongst the following the most basic compound is :
(A) p-nitroaniline
(B) acetanilide
(C) aniline
(D) benzylamine
Answer
548.1k+ views
Hint: Among the following given compounds, basicity is determined by the availability of lone pairs. If the lone pair donation tendency is more, then basicity is more. If resonance is more, then the tendency to donate lone pairs is less.
Complete Step by step solution:
All the given compounds have a benzene ring present within them. As the benzene ring is present, resonance takes place. Resonance involves delocalization of electrons within the molecules. Resonating structures or canonical structures are contributory structures which together form the resonance hybrid. Greater the resonance, greater is the stability, less is the tendency to donate a lone pair and less basicity.
The structure of p-nitroaniline is as follows,
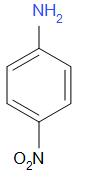
P-nitroaniline is also known as 4-nitroaniline. It is an organic compound with formula $ {C_6}{H_6}{N_2}{O_2} $ . It is used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline. In p-nitroaniline, the lone pair of nitrogen in $ - N{H_2} $ is in resonance with the benzene ring.
The structure of acetanilide is as follows,
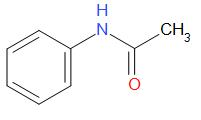
Acetanilide is an odourless, solid chemical compound. It is also known as acetanil. It is used as an indicator of hydrogen peroxide decomposition. Acetanilide possesses analgesic as well as antipyretic properties. In acetanilide, the lone pair on nitrogen is again in resonance.
The structure of aniline is as follows,
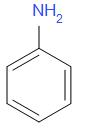
The biggest application of aniline is for the preparation of methylenedianiline. The smell of aniline is like rotten fish. It ignites readily and burns with a smoky flame. The lone pair on nitrogen is again in resonance here.
The structure of benzylamine is as follows,
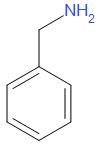
Benzylamine is a colourless, water-soluble liquid. Benzylamine takes part in the Schotten-Baumann reaction. Benzylamine is used in the industrial manufacturing of numerous pharmaceuticals. Here, the lone pair of nitrogen is not involved in resonance.
Of the following compounds, the lone pair of nitrogen is not involved in resonance in benzylamine. Hence, it can donate lone pairs easily as compared to others. So, among the following the most basic compound is benzylamine.
Therefore, the correct answer is option D.
Note:
Resonance effect or mesomeric effect is a permanent effect. It is more prominent in aryl compounds. It is the main reason for stability of these compounds. More the resonance, more is the stability.
Complete Step by step solution:
All the given compounds have a benzene ring present within them. As the benzene ring is present, resonance takes place. Resonance involves delocalization of electrons within the molecules. Resonating structures or canonical structures are contributory structures which together form the resonance hybrid. Greater the resonance, greater is the stability, less is the tendency to donate a lone pair and less basicity.
The structure of p-nitroaniline is as follows,

P-nitroaniline is also known as 4-nitroaniline. It is an organic compound with formula $ {C_6}{H_6}{N_2}{O_2} $ . It is used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline. In p-nitroaniline, the lone pair of nitrogen in $ - N{H_2} $ is in resonance with the benzene ring.
The structure of acetanilide is as follows,

Acetanilide is an odourless, solid chemical compound. It is also known as acetanil. It is used as an indicator of hydrogen peroxide decomposition. Acetanilide possesses analgesic as well as antipyretic properties. In acetanilide, the lone pair on nitrogen is again in resonance.
The structure of aniline is as follows,

The biggest application of aniline is for the preparation of methylenedianiline. The smell of aniline is like rotten fish. It ignites readily and burns with a smoky flame. The lone pair on nitrogen is again in resonance here.
The structure of benzylamine is as follows,

Benzylamine is a colourless, water-soluble liquid. Benzylamine takes part in the Schotten-Baumann reaction. Benzylamine is used in the industrial manufacturing of numerous pharmaceuticals. Here, the lone pair of nitrogen is not involved in resonance.
Of the following compounds, the lone pair of nitrogen is not involved in resonance in benzylamine. Hence, it can donate lone pairs easily as compared to others. So, among the following the most basic compound is benzylamine.
Therefore, the correct answer is option D.
Note:
Resonance effect or mesomeric effect is a permanent effect. It is more prominent in aryl compounds. It is the main reason for stability of these compounds. More the resonance, more is the stability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




