
Among\[Ni{{(CO)}_{4}},{{[Ni{{(CN)}_{4}}]}^{2-}}\], and \[NiCl_{4}^{2-}\]:
(a) \[Ni{{(CO)}_{4}}\] and \[NiCl_{4}^{2-}\] are diamagnetic and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] is paramagnetic
(b) \[NiCl_{4}^{2-}\] and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] are diamagnetic and \[Ni{{(CO)}_{4}}\] is paramagnetic
(c) \[Ni{{(CO)}_{4}}\] and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] are diamagnetic and \[NiCl_{4}^{2-}\] is paramagnetic
(d) \[Ni{{(CO)}_{4}}\] is diamagnetic, and \[NiCl_{4}^{2-}\] and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] are paramagnetic
Answer
594.9k+ views
Hint: When there are no unpaired electrons present in an ion or molecule, it is diamagnetic. If there are unpaired electrons, then the molecule or ion is paramagnetic, that is it shows some magnetism.
Complete answer:
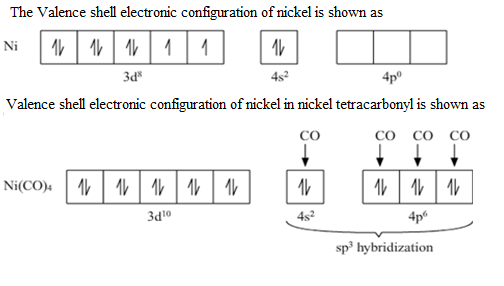
-\[Ni{{(CO)}_{4}}\] consists of Ni and four carbonyl ligands.
The valence shell electronic configuration of Ni atom will be \[3{{d}^{8}}4{{s}^{2}}\]
All the 10 electrons in the valence shell are pushed into a 3d orbital and they get paired up due to the strong field of CO ligand approach Ni atom (CO ligand is strong field ligand due to the presence of pi back bonding due to this it has the ability to pair up the unpaired electrons of the metal d orbital). The empty 4s and 4p orbital then undergo \[s{{p}^{3}}\] hybridisation and form bonds with the CO ligands giving the compound \[Ni{{(CO)}_{4}}\]. So, there are no unpaired electrons in the compound, hence, it is diamagnetic.
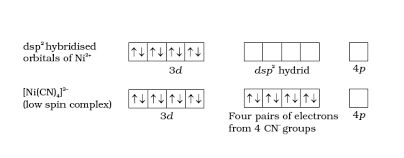
-\[{{[Ni{{(CN)}_{4}}]}^{2-}}\] has nickel in +2 oxidation state and its electronic configuration in the valence shell is \[3{{d}^{8}}4{{s}^{0}}\]
Due to the presence of strong ligands \[C{{N}^{-}}\], all the electrons are paired up. So, there will be empty 4d, 3s and two 4p orbitals which undergo \[ds{{p}^{2}}\] hybridisation and make bonds with \[C{{N}^{-}}\] ligands and attain square planar geometry. Due to the presence of unpaired electrons it is diamagnetic.
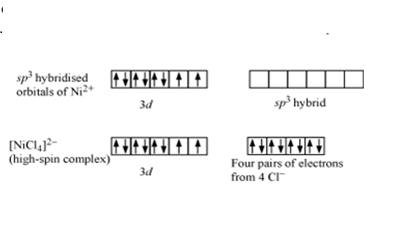
-In \[NiCl_{4}^{2-}\] Ni is in +2 oxidation state and the valence shell electronic configuration is \[3{{d}^{8}}4{{s}^{0}}\]. Here chloride ions are weak field ligands. They don’t have the capability to pair up the unpaired electrons in the valence shell electrons. Therefore, no pairing of d electrons occur. \[N{{i}^{2+}}\] undergoes \[s{{p}^{3}}\] hybridisation and makes bonds with chloride ligands in tetrahedral geometry. Due to the presence of unpaired electrons, \[NiCl_{4}^{2-}\] is paramagnetic and is referred to as a high spin complex.
Hence, the correct answer is \[Ni{{(CO)}_{4}}\] and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] are diamagnetic and \[NiCl_{4}^{2-}\] is paramagnetic. That is option (c).
Note: We should always check that the ligands with which the complex is made of is strong field ligand or weak field ligand. Strong field ligands can pair up the unpaired electrons in an orbital, while weak field ligands do not.
Complete answer:
-\[Ni{{(CO)}_{4}}\] consists of Ni and four carbonyl ligands.
The valence shell electronic configuration of Ni atom will be \[3{{d}^{8}}4{{s}^{2}}\]
All the 10 electrons in the valence shell are pushed into a 3d orbital and they get paired up due to the strong field of CO ligand approach Ni atom (CO ligand is strong field ligand due to the presence of pi back bonding due to this it has the ability to pair up the unpaired electrons of the metal d orbital). The empty 4s and 4p orbital then undergo \[s{{p}^{3}}\] hybridisation and form bonds with the CO ligands giving the compound \[Ni{{(CO)}_{4}}\]. So, there are no unpaired electrons in the compound, hence, it is diamagnetic.

-\[{{[Ni{{(CN)}_{4}}]}^{2-}}\] has nickel in +2 oxidation state and its electronic configuration in the valence shell is \[3{{d}^{8}}4{{s}^{0}}\]
Due to the presence of strong ligands \[C{{N}^{-}}\], all the electrons are paired up. So, there will be empty 4d, 3s and two 4p orbitals which undergo \[ds{{p}^{2}}\] hybridisation and make bonds with \[C{{N}^{-}}\] ligands and attain square planar geometry. Due to the presence of unpaired electrons it is diamagnetic.

-In \[NiCl_{4}^{2-}\] Ni is in +2 oxidation state and the valence shell electronic configuration is \[3{{d}^{8}}4{{s}^{0}}\]. Here chloride ions are weak field ligands. They don’t have the capability to pair up the unpaired electrons in the valence shell electrons. Therefore, no pairing of d electrons occur. \[N{{i}^{2+}}\] undergoes \[s{{p}^{3}}\] hybridisation and makes bonds with chloride ligands in tetrahedral geometry. Due to the presence of unpaired electrons, \[NiCl_{4}^{2-}\] is paramagnetic and is referred to as a high spin complex.

Hence, the correct answer is \[Ni{{(CO)}_{4}}\] and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] are diamagnetic and \[NiCl_{4}^{2-}\] is paramagnetic. That is option (c).
Note: We should always check that the ligands with which the complex is made of is strong field ligand or weak field ligand. Strong field ligands can pair up the unpaired electrons in an orbital, while weak field ligands do not.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




