
Among these cations, which of the following orders is correct for their no- bond- resonance energy?
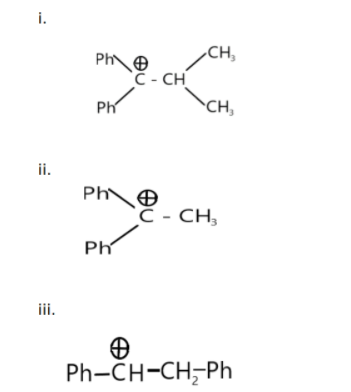
a. i > ii > iii
b. iii > i > ii
c. iii > ii > i
d. ii > iii > i

Answer
569.4k+ views
Hint: When there is a positive charge on carbon the compound is generally called carbocation, all the structure has 1 carbocation, no – bond – resonance is called hyperconjugation, where the sigma or lone pair electrons move to the adjacent pi orbital.
Complete Step by step answer:First, let’s understand the role of hyperconjugation in a molecule?
Hyperconjugation or no – bond – resonance brings stability to the molecule, the more the extent of hyperconjugation the more will be the stability of the corresponding molecule.
Hyperconjugation is directly proportional to the number of $\alpha $ hydrogen present in the given molecule. Hence, the greater the number of $\alpha $ hydrogen greater will be the hyperconjugation or non-bond- resonance energy.
The carbon adjacent to the function group is called $\alpha $ carbon, and the hydrogen attached to the $\alpha $ carbon is called $\alpha $ hydrogen.
Therefore, to choose the correct option we have to count the $\alpha $ hydrogen present in each molecule.
The maximum number of $\alpha $ hydrogen is present in molecule ‘ii’ and followed by 2 and 1 in molecule ‘iii and i’ respectively. The order of hyperconjugation can be written as ii > iii > i
Hence, the correct answer is option (d) i.e., ii > iii > i.
Note: The phenyl group ($ - {C_6}{H_5}$) has 5 hydrogens but these hydrogen are not $\alpha $ hydrogen as the $\alpha $ carbon bonded with carbocation has no hydrogen in it. The functional group is usually alkene, but here one hydrogen from one of the alkene carbon is removed, leaving a + ve charge behind. Hence, we will consider the carbocation ($\mathop C\limits^ \oplus $) as the functional group.
Complete Step by step answer:First, let’s understand the role of hyperconjugation in a molecule?
Hyperconjugation or no – bond – resonance brings stability to the molecule, the more the extent of hyperconjugation the more will be the stability of the corresponding molecule.
Hyperconjugation is directly proportional to the number of $\alpha $ hydrogen present in the given molecule. Hence, the greater the number of $\alpha $ hydrogen greater will be the hyperconjugation or non-bond- resonance energy.
The carbon adjacent to the function group is called $\alpha $ carbon, and the hydrogen attached to the $\alpha $ carbon is called $\alpha $ hydrogen.
Therefore, to choose the correct option we have to count the $\alpha $ hydrogen present in each molecule.
The maximum number of $\alpha $ hydrogen is present in molecule ‘ii’ and followed by 2 and 1 in molecule ‘iii and i’ respectively. The order of hyperconjugation can be written as ii > iii > i
Hence, the correct answer is option (d) i.e., ii > iii > i.
Note: The phenyl group ($ - {C_6}{H_5}$) has 5 hydrogens but these hydrogen are not $\alpha $ hydrogen as the $\alpha $ carbon bonded with carbocation has no hydrogen in it. The functional group is usually alkene, but here one hydrogen from one of the alkene carbon is removed, leaving a + ve charge behind. Hence, we will consider the carbocation ($\mathop C\limits^ \oplus $) as the functional group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




