
Among the oxides of nitrogen ${{\text{N}}_{2}}{{\text{O}}_{3}}\text{, }{{\text{N}}_{2}}{{\text{O}}_{4}}\text{ and }{{\text{N}}_{2}}{{\text{O}}_{5}}$;molecule(s) having nitrogen-nitrogen bond is/are:
$\begin{align}
& \text{A}\text{. Only }{{\text{N}}_{2}}{{\text{O}}_{5}} \\
& \text{B}\text{. }{{\text{N}}_{2}}{{\text{O}}_{3}}\text{ and }{{\text{N}}_{2}}{{\text{O}}_{4}} \\
& \text{C}\text{. }{{\text{N}}_{2}}{{\text{O}}_{3}}\text{ }\text{and }{{\text{N}}_{2}}{{\text{O}}_{5}} \\
& \text{D}\text{. }{{\text{N}}_{2}}{{\text{O}}_{4}}\text{ and }{{\text{N}}_{2}}{{\text{O}}_{5}} \\
\end{align}$
Answer
568.2k+ views
Hint: Electronic configuration of the nitrogen is \[\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{3}}\] due to which it can form 3 bonds easily with other atoms. The electronegativity of oxygen is more than the nitrogen because of its larger size than nitrogen. Electronegativity is the tendency of an element to attract a pair of electrons from another element.
Complete Solution:
-We have to identify the N - N bond from the given three compounds of nitrogen oxide which are also known as acidic oxides.
-So, firstly we have to draw the structure of nitrogen trioxide, nitrogen tetroxide and nitrogen penta-oxide which have molecular formula of ${{\text{N}}_{2}}{{\text{O}}_{3}}\text{, }{{\text{N}}_{2}}{{\text{O}}_{4}}\text{ and }{{\text{N}}_{2}}{{\text{O}}_{5}}$ respectively.
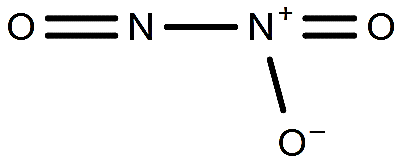
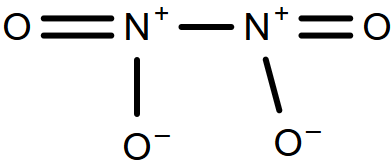
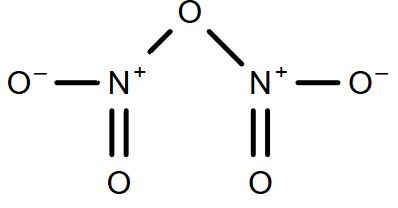
-So, we can see that only ${{\text{N}}_{2}}{{\text{O}}_{3}}$ and ${{\text{N}}_{2}}{{\text{O}}_{4}}$ have only one N-N bond.
-In ${{\text{N}}_{2}}{{\text{O}}_{5}}$, there is 3 N - O and 2 N = O bond and not N-N bond so it will be the incorrect answer.
-The name of the compound ${{\text{N}}_{2}}{{\text{O}}_{3}}$ is nitrogen trioxide and it is formed when the nitrogen oxide and nitrogen dioxide reacts together i.e. \[\text{NO + N}{{\text{O}}_{2}}\to \text{ }{{\text{N}}_{2}}{{\text{O}}_{3}}\].
-Whereas when two molecules of nitrogen oxide dimerise with each other they form nitrogen tetroxide i.e. \[2\text{N}{{\text{O}}_{2}}\ \rightleftharpoons \text{ }{{\text{N}}_{2}}{{\text{O}}_{4}}\]
-Moreover, the oxidation state of nitrogen in nitrogen trioxide, nitrogen tetroxide and nitrogen penta-oxide is +2, +3 and +5.
-So, only nitrogen trioxide and nitrogen tetroxide will have a N - N bond.
Note: Nitrogen can form maximum of 3 covalent bond and 1 co-ordinate bond because according to the electronic configuration it has ability to form 3 bonds easily but in some cases, it can also share some a pair electron i.e. lone pair which forms coordination bond.
Complete Solution:
-We have to identify the N - N bond from the given three compounds of nitrogen oxide which are also known as acidic oxides.
-So, firstly we have to draw the structure of nitrogen trioxide, nitrogen tetroxide and nitrogen penta-oxide which have molecular formula of ${{\text{N}}_{2}}{{\text{O}}_{3}}\text{, }{{\text{N}}_{2}}{{\text{O}}_{4}}\text{ and }{{\text{N}}_{2}}{{\text{O}}_{5}}$ respectively.
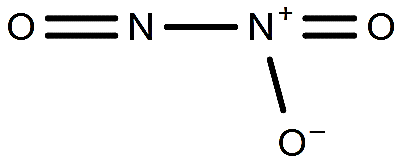
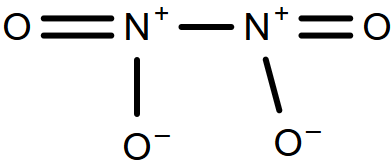

-So, we can see that only ${{\text{N}}_{2}}{{\text{O}}_{3}}$ and ${{\text{N}}_{2}}{{\text{O}}_{4}}$ have only one N-N bond.
-In ${{\text{N}}_{2}}{{\text{O}}_{5}}$, there is 3 N - O and 2 N = O bond and not N-N bond so it will be the incorrect answer.
-The name of the compound ${{\text{N}}_{2}}{{\text{O}}_{3}}$ is nitrogen trioxide and it is formed when the nitrogen oxide and nitrogen dioxide reacts together i.e. \[\text{NO + N}{{\text{O}}_{2}}\to \text{ }{{\text{N}}_{2}}{{\text{O}}_{3}}\].
-Whereas when two molecules of nitrogen oxide dimerise with each other they form nitrogen tetroxide i.e. \[2\text{N}{{\text{O}}_{2}}\ \rightleftharpoons \text{ }{{\text{N}}_{2}}{{\text{O}}_{4}}\]
-Moreover, the oxidation state of nitrogen in nitrogen trioxide, nitrogen tetroxide and nitrogen penta-oxide is +2, +3 and +5.
-So, only nitrogen trioxide and nitrogen tetroxide will have a N - N bond.
Note: Nitrogen can form maximum of 3 covalent bond and 1 co-ordinate bond because according to the electronic configuration it has ability to form 3 bonds easily but in some cases, it can also share some a pair electron i.e. lone pair which forms coordination bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




