
Among the following, Vander waals’ forces are maximum in:
A. $HBr$
B. $LiBr$
C. $LiCl$
D. $AgBr$
Answer
572.1k+ views
Hint:Van der waals forces are specific intermolecular interactions observed generally in solids and liquids. They are electrostatic in nature. Van Der waals interaction arises from the interactions of positively and negatively charged species
Complete answer:
Intramolecular forces hold atoms within molecules together for example covalent or ionic bonds. But vander waal forces are the intermolecular forces that hold the molecules together. Vander waals forces include attraction and repulsion between atoms, molecules and surfaces. These forces help in determining physical properties like boiling point or melting point. London dispersion force, dipole-dipole interaction and hydrogen force are collectively known as vander waal force.
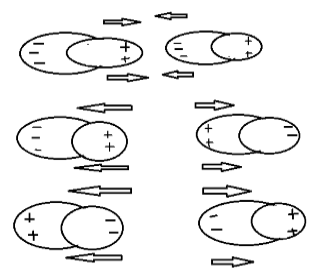
As we know, positive charges repel each other, negative charges also repel each other but positive charge and negative charge attract each other. As shown above.
In atoms, the electrons are continuously orbiting in shells. It is possible that at some point all the electrons come to one side of the atom, making it an instantaneous dipole that repels the electrons of neighboring atoms, making an induced dipole. This interaction between instantaneous dipole-induced dipole is known as the London dispersion force
Dipole-dipole forces are similar to London dispersion forces, but they occur in molecules that are permanently polar versus momentarily polar. . The ability of a molecule to become polar and displace its electron is known as polarisability. The more electrons a molecule contains, the higher its ability to become polar. when the molecules become polar the London dispersion forces and dipole-dipole interactions increase and hence vander waal forces increase between them.
Vander waal forces are maximum in ionic compounds. Vander waal forces also depend on the number of electrons present in the molecule, hence $AgBr$ have strong van der waal’s force.
Correct option is D.
Note:
Being the weakest of the chemical forces, they still support an integral structural load when many of these interactions are present. The main characteristics of vander waal forces are that they are weaker than normal covalent and ionic bonds. They are additives. They are short range forces hence when the distance increases these forces vanishes.
Complete answer:
Intramolecular forces hold atoms within molecules together for example covalent or ionic bonds. But vander waal forces are the intermolecular forces that hold the molecules together. Vander waals forces include attraction and repulsion between atoms, molecules and surfaces. These forces help in determining physical properties like boiling point or melting point. London dispersion force, dipole-dipole interaction and hydrogen force are collectively known as vander waal force.

As we know, positive charges repel each other, negative charges also repel each other but positive charge and negative charge attract each other. As shown above.
In atoms, the electrons are continuously orbiting in shells. It is possible that at some point all the electrons come to one side of the atom, making it an instantaneous dipole that repels the electrons of neighboring atoms, making an induced dipole. This interaction between instantaneous dipole-induced dipole is known as the London dispersion force
Dipole-dipole forces are similar to London dispersion forces, but they occur in molecules that are permanently polar versus momentarily polar. . The ability of a molecule to become polar and displace its electron is known as polarisability. The more electrons a molecule contains, the higher its ability to become polar. when the molecules become polar the London dispersion forces and dipole-dipole interactions increase and hence vander waal forces increase between them.
Vander waal forces are maximum in ionic compounds. Vander waal forces also depend on the number of electrons present in the molecule, hence $AgBr$ have strong van der waal’s force.
Correct option is D.
Note:
Being the weakest of the chemical forces, they still support an integral structural load when many of these interactions are present. The main characteristics of vander waal forces are that they are weaker than normal covalent and ionic bonds. They are additives. They are short range forces hence when the distance increases these forces vanishes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




