
Among the following the number of aromatic compound(s) is/are:

Answer
529.4k+ views
Hint: An aromatic compound is one that has high stability and some properties due to closed loop of electrons. Not all molecules with a ring or loop structures are aromatic. Aromatic molecules are sometimes referred to as aromatics.
Complete answer:
In organic chemistry, definition of aromatic compound is given by Huckel’s rule.
Huckel’s rule state, for a compound to be aromatic, compound should be planar, cyclic and should have \[\left( {{{4n + 2}}} \right){{\,\pi }}\]electrons that should be in continuous delocalization or an uninterrupted cyclic pi electron cloud should be there. Here \[{{n = 0,1,2 \ldots \ldots }}\]
So if we put \[{{n = 0}}\] , we get ${{2\,\pi }}$ electrons
If we put \[{{n = 1}}\] , we get ${{6\,\pi }}$ electrons
If we put \[{{n = 2}}\] , we get ${{10\,\pi }}$ electrons and so on
For example benzene, we all know are aware of the structure of benzene. It is planar, it is cyclic and it has ${{6\,\pi }}$ electrons (\[\text{for n = 1 we get, 4} \times \text{1 + 2 = 6}\])
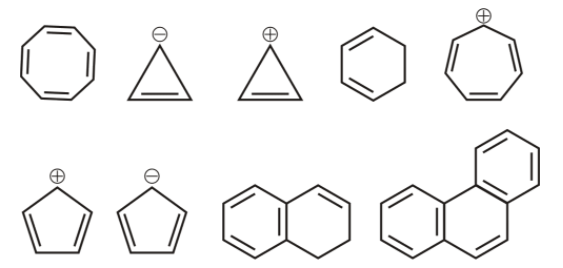
1st we have cyclooctatetraene ,
it is cyclic, and non-planar and the number of ${{\,\pi }}$ electrons are 8, it does not follow huckel’s rule, hence it is not aromatic.
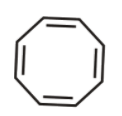
2nd we have cyclopropane anion
It is cyclic, planar and has ${{4\,\pi }}$ electrons and Is not following huckel’s rule hence not aromatic.
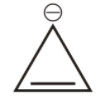
3rd we have cyclopropane cation.
It is cyclic, but it has only ${{2\,\pi }}$ electron, hence obey huckel’s rule hence is aromatic
4th we have cyclohexa-1,3-diene
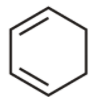
It is cyclic, planar but has ${{4\,\pi }}$ electron. Do not obey huckel’s rule . hence is not aromatic.
5th we have, cyclohepta-1,3,5-triene cation,
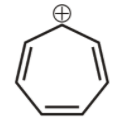
It is cyclic, planar, and has ${{6\,\pi }}$ electron and because of + charge the pi electrons are in continuous delocalization, hence this compound is aromatic.
6th we have cyclopenta-1,3-diene cation,
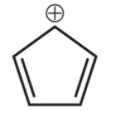
It is cyclic, not planar. It does not obey huckel’s rule. hence is not aromatic
7th we have cyclopenta-1,3-diene anion,
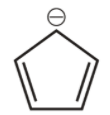
It is cyclic, planar and has ${{6\,\pi }}$ conjugated electrons that means all the pi electrons are in continuous delocalization hence we consider negative charge in conjugation. Hence it is aromatic.
8th we have
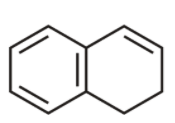
It is cyclic, planar but the electron cloud is not in conjugation. Hence is not aromatic.
9th we have phenanthrene
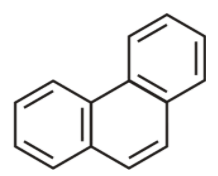
It is cyclic, planar and has ${{14\,\pi }}$ electron which are in conjugation. Hence it does obey huckel’s rule . Hence it is aromatic.
Hence the total number of aromatic compounds is 4.
Note:
Molecules which are cyclic, planar, and have ${{4n\,\pi }}$ electrons which are in conjugation are called anti-aromatic. The compounds which do not follow huckel’s rule for aromaticity and anti-aromaticity are non-aromatic. Aromatic compounds generally have distinctive aroma.
Complete answer:
In organic chemistry, definition of aromatic compound is given by Huckel’s rule.
Huckel’s rule state, for a compound to be aromatic, compound should be planar, cyclic and should have \[\left( {{{4n + 2}}} \right){{\,\pi }}\]electrons that should be in continuous delocalization or an uninterrupted cyclic pi electron cloud should be there. Here \[{{n = 0,1,2 \ldots \ldots }}\]
So if we put \[{{n = 0}}\] , we get ${{2\,\pi }}$ electrons
If we put \[{{n = 1}}\] , we get ${{6\,\pi }}$ electrons
If we put \[{{n = 2}}\] , we get ${{10\,\pi }}$ electrons and so on
For example benzene, we all know are aware of the structure of benzene. It is planar, it is cyclic and it has ${{6\,\pi }}$ electrons (\[\text{for n = 1 we get, 4} \times \text{1 + 2 = 6}\])
1st we have cyclooctatetraene ,

it is cyclic, and non-planar and the number of ${{\,\pi }}$ electrons are 8, it does not follow huckel’s rule, hence it is not aromatic.
2nd we have cyclopropane anion

It is cyclic, planar and has ${{4\,\pi }}$ electrons and Is not following huckel’s rule hence not aromatic.
3rd we have cyclopropane cation.

It is cyclic, but it has only ${{2\,\pi }}$ electron, hence obey huckel’s rule hence is aromatic
4th we have cyclohexa-1,3-diene

It is cyclic, planar but has ${{4\,\pi }}$ electron. Do not obey huckel’s rule . hence is not aromatic.
5th we have, cyclohepta-1,3,5-triene cation,

It is cyclic, planar, and has ${{6\,\pi }}$ electron and because of + charge the pi electrons are in continuous delocalization, hence this compound is aromatic.
6th we have cyclopenta-1,3-diene cation,

It is cyclic, not planar. It does not obey huckel’s rule. hence is not aromatic
7th we have cyclopenta-1,3-diene anion,

It is cyclic, planar and has ${{6\,\pi }}$ conjugated electrons that means all the pi electrons are in continuous delocalization hence we consider negative charge in conjugation. Hence it is aromatic.
8th we have

It is cyclic, planar but the electron cloud is not in conjugation. Hence is not aromatic.
9th we have phenanthrene

It is cyclic, planar and has ${{14\,\pi }}$ electron which are in conjugation. Hence it does obey huckel’s rule . Hence it is aromatic.
Hence the total number of aromatic compounds is 4.
Note:
Molecules which are cyclic, planar, and have ${{4n\,\pi }}$ electrons which are in conjugation are called anti-aromatic. The compounds which do not follow huckel’s rule for aromaticity and anti-aromaticity are non-aromatic. Aromatic compounds generally have distinctive aroma.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




