
Among the following molecule possessing highest dipole moment is:
A ) \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\]
B ) \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\]
C ) \[{\rm{S}}{{\rm{O}}_{\rm{2}}}\]
D ) trans-2-butene
Answer
590.4k+ views
Hint: Dipole moment is the product of the charge and the distance between the centers of positive and negative charges. Determine whether each molecule is symmetrical or not and also determine if the atoms have significant electronegativity difference.
Complete answer:
> If a bond is present between two atoms having same electronegativity, then the bond is nonpolar. A bond is polar if the atoms have an electronegativity difference. If a molecule contains only nonpolar bonds, then it is non-polar with zero dipole moment. If one or more polar bonds are present in the molecule, then the polar or nonpolar nature of the molecule depends on its molecular symmetry.
> If an asymmetrical molecule contains polar bonds, then the molecule has a dipole moment. In this molecule, the individual bond dipoles do not cancel each other. Due to this, the net (resultant) dipole moment of the molecule is non zero.
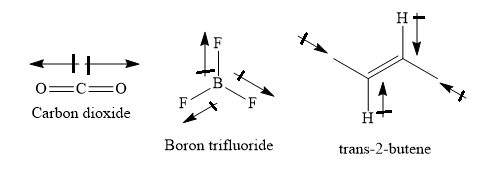
The structures of sulphur dioxide, carbon dioxide, boron trifluoride and trans-2-butene are as shown below:
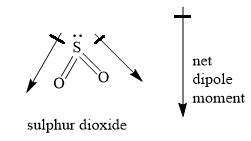
> Sulphur dioxide contains two elements, sulphur and oxygen. The electronegativity value of sulphur is significantly different from the electronegativity value of oxygen. Hence, the bond between sulphur and oxygen is polar. The central sulphur atom has two bonding domains and one lone pair of electrons. \[s{p^2}\] hybridisation results in electron pair geometry of trigonal planar and molecular geometry of bent or V shaped. Sulphur dioxide molecule is an asymmetrical molecule containing polar bonds. The individual bond dipoles do not cancel each other and the molecule is polar with non zero dipole moment.
> Carbon dioxide, boron trifluoride and trans-2-butene are symmetrical molecules with zero dipole moment. In these molecules, the individual bond dipoles cancel each other.
Sulphur dioxide molecules have the highest dipole moment.
Hence, the option C ) is the correct answer.
Note: When a system has charge separation, the dipole moment arises. Both ionic and covalent bonds possess dipole moments. The bond dipole moment is a vector quantity, as it has magnitude and direction. When several polar bonds are present in the molecule, the net dipole moment of the molecule is the vector addition of these individual bond dipole vectors.
Complete answer:
> If a bond is present between two atoms having same electronegativity, then the bond is nonpolar. A bond is polar if the atoms have an electronegativity difference. If a molecule contains only nonpolar bonds, then it is non-polar with zero dipole moment. If one or more polar bonds are present in the molecule, then the polar or nonpolar nature of the molecule depends on its molecular symmetry.
> If an asymmetrical molecule contains polar bonds, then the molecule has a dipole moment. In this molecule, the individual bond dipoles do not cancel each other. Due to this, the net (resultant) dipole moment of the molecule is non zero.
The structures of sulphur dioxide, carbon dioxide, boron trifluoride and trans-2-butene are as shown below:


> Sulphur dioxide contains two elements, sulphur and oxygen. The electronegativity value of sulphur is significantly different from the electronegativity value of oxygen. Hence, the bond between sulphur and oxygen is polar. The central sulphur atom has two bonding domains and one lone pair of electrons. \[s{p^2}\] hybridisation results in electron pair geometry of trigonal planar and molecular geometry of bent or V shaped. Sulphur dioxide molecule is an asymmetrical molecule containing polar bonds. The individual bond dipoles do not cancel each other and the molecule is polar with non zero dipole moment.
> Carbon dioxide, boron trifluoride and trans-2-butene are symmetrical molecules with zero dipole moment. In these molecules, the individual bond dipoles cancel each other.
Sulphur dioxide molecules have the highest dipole moment.
Hence, the option C ) is the correct answer.
Note: When a system has charge separation, the dipole moment arises. Both ionic and covalent bonds possess dipole moments. The bond dipole moment is a vector quantity, as it has magnitude and direction. When several polar bonds are present in the molecule, the net dipole moment of the molecule is the vector addition of these individual bond dipole vectors.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




