
Among the following given compounds, the one that is polar and has a central atom with $s{p^3}$ hybridization is:
A. ${H_2}C{O_3}$
B. $Si{F_4}$
C. $B{F_3}$
D. $HCl{O_2}$
Answer
578.4k+ views
Hint:In $s{p^3}$ hybridization mixing of one 2s-orbital and three 2p-orbital takes place to form four hybrid orbitals. In order for a molecule to possess $s{p^3}$ hybridization, it should contain one s-orbital and three p orbital. The shape of the compound of $s{p^3}$ hybridization is tetrahedral.
Complete step by step answer:
The polarity of the compound is dependent on the ability of an atom present in a compound to attract another atom. Due to this attraction, there is a difference in electronegativity when one atom pulls the other atom more strongly and then the compound is said to be polar in nature.
${H_2}C{O_3}$
The structure of ${H_2}C{O_3}$ is shown below.
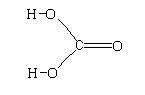
The structure shows that it contains a 3 sigma bond and 1 pi bond.
The hybridization is $s{p^2}$ and the structure is trigonal planar.
The compound is polar as there is an electronegativity difference between the carbon atom and oxygen atom.
$Si{F_4}$
The structure of $Si{F_4}$ is shown below.
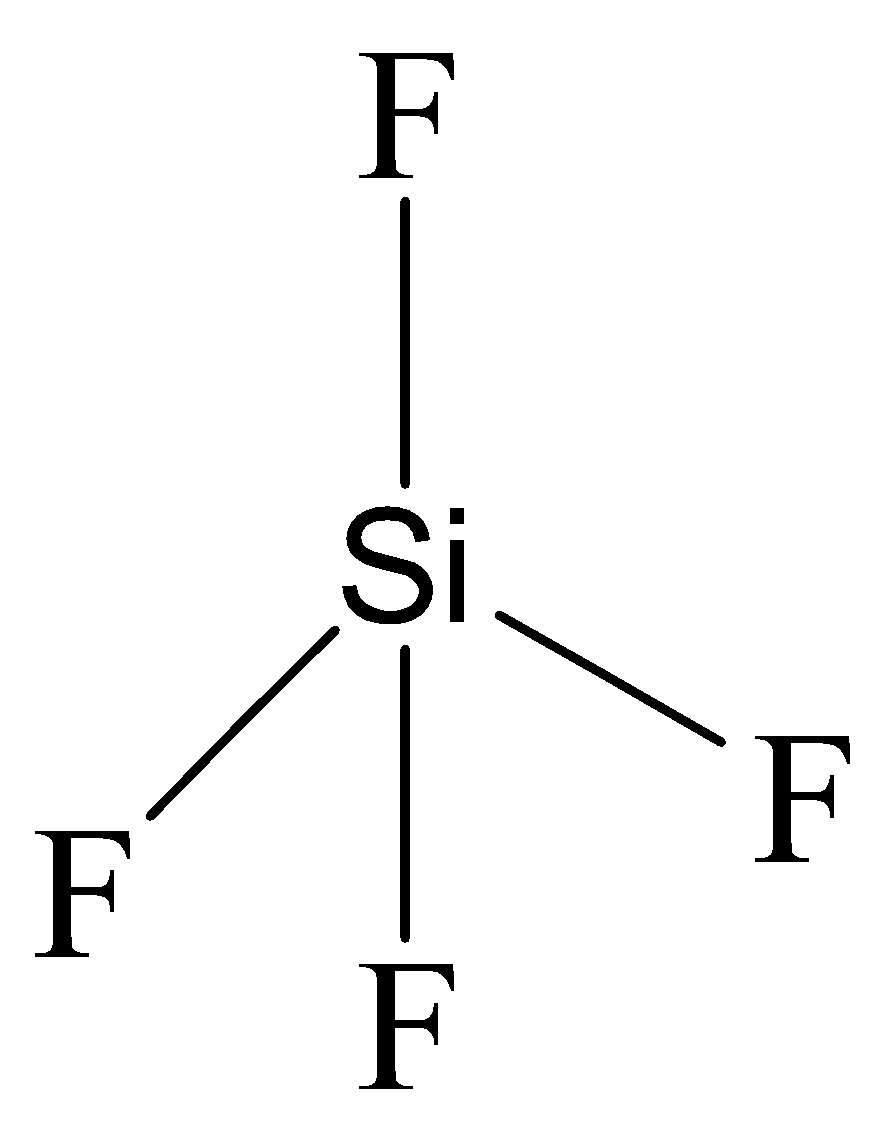
It contains four sigma bonds and the geometry is tetrahedral. The hybridization is $s{p^3}$. Fluorine is a very electronegative element, the dipole moment of the four Si-F bonds will cancel out and the resulting dipole moment will be zero. Thus it is not a polar compound.
$B{F_3}$
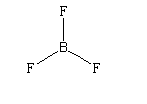
It contains three sigma bonds and the geometry is trigonal planar. The hybridization is $s{p^2}$. The net dipole moment is zero. Thus it is not a polar compound.
$HCl{O_2}$
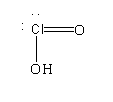
It contains three bond pairs and two lone pairs. The hybridization is $s{p^3}$. It is a polar compound.
Therefore, the correct option is D.
Note:
The lone pairs also take part in hybridization as it is localized at the central metal in pure orbital and the orbitals of the metal atom take part in hybridization.
Complete step by step answer:
The polarity of the compound is dependent on the ability of an atom present in a compound to attract another atom. Due to this attraction, there is a difference in electronegativity when one atom pulls the other atom more strongly and then the compound is said to be polar in nature.
${H_2}C{O_3}$
The structure of ${H_2}C{O_3}$ is shown below.

The structure shows that it contains a 3 sigma bond and 1 pi bond.
The hybridization is $s{p^2}$ and the structure is trigonal planar.
The compound is polar as there is an electronegativity difference between the carbon atom and oxygen atom.
$Si{F_4}$
The structure of $Si{F_4}$ is shown below.

It contains four sigma bonds and the geometry is tetrahedral. The hybridization is $s{p^3}$. Fluorine is a very electronegative element, the dipole moment of the four Si-F bonds will cancel out and the resulting dipole moment will be zero. Thus it is not a polar compound.
$B{F_3}$

It contains three sigma bonds and the geometry is trigonal planar. The hybridization is $s{p^2}$. The net dipole moment is zero. Thus it is not a polar compound.
$HCl{O_2}$

It contains three bond pairs and two lone pairs. The hybridization is $s{p^3}$. It is a polar compound.
Therefore, the correct option is D.
Note:
The lone pairs also take part in hybridization as it is localized at the central metal in pure orbital and the orbitals of the metal atom take part in hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




