
Among the elements from atomic number \[1\] to \[36\] , the number of elements which have an unpaired electron in their s-subshell is:
A. $7$
B. $9$
C. $4$
D. $6$
Answer
573.9k+ views
Hint:In order to answer this question one should be able to write the electronic configuration of the elements asked in the question. The electronic configuration tells us how the electrons are distributed in the orbitals, in order to write an electronic configuration the below diagram should be kept in mind:
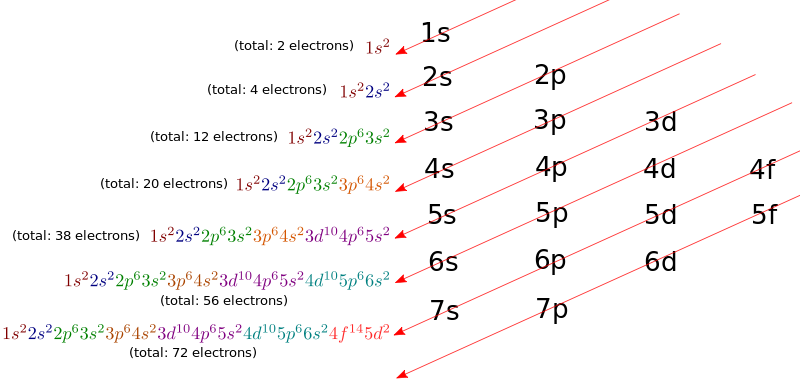
Complete step by step answer:
Below is the list of electronic configuration of elements from $1$ to $36$, it has been written in shorthand electron configuration, i.e., starting with the noble gas symbol in the previous period, followed by the remaining configuration
From the table we can clearly see that only elements (hydrogen, lithium, beryllium, sodium, copper and chromium) have unpaired electrons in the s subshell.
Hence Option D is correct.
Note:
It is important to note here that there are two exceptions, Copper and Chromium, in the expected electronic configuration of copper it should have been \[\left[ {Ar} \right]3{d^9}4{s^2}\] but because a fully filled $d$ sublevel is more stable than a partially filled one, therefore an electron from the $4s$ orbital gets excited and $3d$ orbital giving us the configuration mentioned in the table above.
Same happens in the case of Chromium, where expected configuration would have been \[\left[ {Ar} \right]3{d^4}4{s^2}\] but because half-filled $d$ orbital is more stable than a partially filled one, therefore the electron gets excited and goes to the $d$ orbital giving us the exception implemented electronic configuration.

Complete step by step answer:
Below is the list of electronic configuration of elements from $1$ to $36$, it has been written in shorthand electron configuration, i.e., starting with the noble gas symbol in the previous period, followed by the remaining configuration
| Name of the element | Atomic number | Electronic configuration of the element |
| Hydrogen | $1$ | \[1{s^1}\] |
| Helium | $2$ | \[1{s^2}\] |
| Lithium | $3$ | \[\left[ {He} \right]2{s^1}\] |
| Beryllium | $4$ | \[\left[ {He} \right]2{s^2}\] |
| Boron | $5$ | \[\left[ {He} \right]2{s^2}2{p^1}\] |
| Carbon | $6$ | \[\left[ {He} \right]2{s^2}2{p^2}\] |
| Nitrogen | $7$ | \[\left[ {He} \right]2{s^2}2{p^3}\] |
| Oxygen | $8$ | \[\left[ {He} \right]2{s^2}2{p^4}\] |
| Flourine | $9$ | \[\left[ {He} \right]2{s^2}2{p^5}\] |
| Neon | $10$ | \[\left[ {He} \right]2{s^2}2{p^6}\] |
| Sodium | $11$ | \[\left[ {Ne} \right]3{s^1}\] |
| Magnesium | $12$ | \[\left[ {Ne} \right]3{s^2}\] |
| Aluminium | $13$ | \[\left[ {Ne} \right]3{s^2}3{p^1}\] |
| Silicon | $14$ | \[\left[ {Ne} \right]3{s^2}3{p^2}\] |
| Phosphorus | $15$ | \[\left[ {Ne} \right]3{s^2}3{p^3}\] |
| Sulphur | $16$ | \[\left[ {Ne} \right]3{s^2}3{p^4}\] |
| Chlorine | $17$ | \[\left[ {Ne} \right]3{s^2}3{p^5}\] |
| Argon | $18$ | \[\left[ {Ne} \right]3{s^2}3{p^6}\] |
| Potassium | $19$ | \[\left[ {Ar} \right]4{s^1}\] |
| Calcium | $20$ | \[\left[ {Ar} \right]4{s^2}\] |
| Scandium | $21$ | \[\left[ {Ar} \right]3{d^1}4{s^2}\] |
| Titanium | $22$ | \[\left[ {Ar} \right]3{d^2}4{s^2}\] |
| Vanadium | $23$ | \[\left[ {Ar} \right]3{d^3}4{s^2}\] |
| Chromium | $24$ | \[\left[ {Ar} \right]3{d^5}4{s^1}\] |
| Manganese | $25$ | \[\left[ {Ar} \right]3{d^5}4{s^2}\] |
| Iron | $26$ | \[\left[ {Ar} \right]3{d^6}4{s^2}\] |
| Cobalt | $27$ | \[\left[ {Ar} \right]3{d^7}4{s^2}\] |
| Nickel | $28$ | \[\left[ {Ar} \right]3{d^8}4{s^2}\] |
| Copper | $29$ | \[\left[ {Ar} \right]3{d^{10}}4{s^1}\] |
| Zinc | $30$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}\] |
| Gallium | $31$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^1}\] |
| Germanium | $32$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^2}\] |
| Arsenic | $33$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^3}\] |
| Selenium | $34$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^4}\] |
| Bromine | $35$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^5}\] |
| Krypton | $36$ | \[\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^6}\] |
From the table we can clearly see that only elements (hydrogen, lithium, beryllium, sodium, copper and chromium) have unpaired electrons in the s subshell.
Hence Option D is correct.
Note:
It is important to note here that there are two exceptions, Copper and Chromium, in the expected electronic configuration of copper it should have been \[\left[ {Ar} \right]3{d^9}4{s^2}\] but because a fully filled $d$ sublevel is more stable than a partially filled one, therefore an electron from the $4s$ orbital gets excited and $3d$ orbital giving us the configuration mentioned in the table above.
Same happens in the case of Chromium, where expected configuration would have been \[\left[ {Ar} \right]3{d^4}4{s^2}\] but because half-filled $d$ orbital is more stable than a partially filled one, therefore the electron gets excited and goes to the $d$ orbital giving us the exception implemented electronic configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




