
Among \[[Ni{(CO)_4}]\] , \[{[Ni{(CN)_4}]^{2 - }}\] , \[{[NiC{l_4}]^{2 - }}\] species, the hybridization states at the Ni atom are, respectively (Atomic number of \[Ni = 28\] )
A. \[ds{p^2}\] , \[s{p^3}\] , \[s{p^3}\]
B. \[s{p^3}\] , \[ds{p^2}\] , \[ds{p^2}\]
C. \[s{p^3}\] , \[ds{p^2}\] , \[s{p^3}\]
D. \[s{p^3}\] , \[s{p^3}\] , \[ds{p^2}\]
Answer
581.7k+ views
Hint: Ligands can be understood as ions or molecules which bind with the central atom in order to form a coordination complex. This type of bonding usually takes place when there is a formal donation of one or more pairs of electrons of the ligand. Ligands can be mainly classified into two main types: Strong field ligands and weak field ligands. Let us discuss a little more about these two types of ligands.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Strong field ligands cause a large splitting in the given chemical species. This basically means strong field ligands exert strong ligand electrical fields. This property of strong field ligands makes them capable of forming low spin complexes. This means that strong field ligands disobey Hund’ multiplicity rule and cause pairing of electrons even before the entire subshell is filled with electrons of a specific spin character. On the other hand, weak field ligands obey Hund’s rule.
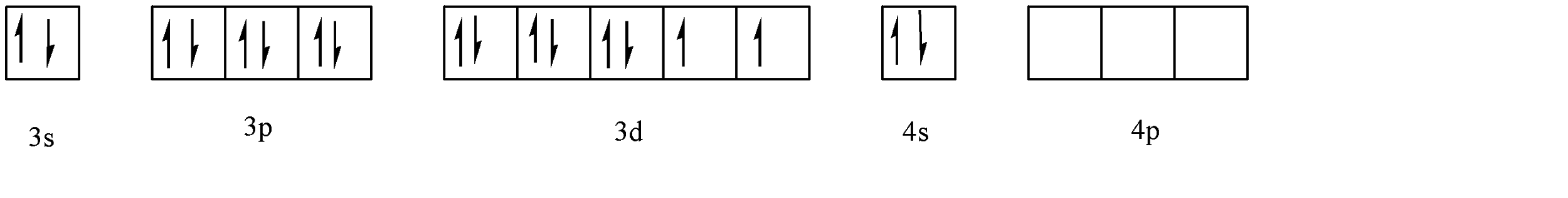
Moving back to the question, we can observe that the ligands attached to the Nickel atom are (CO), (CN) and (Cl). Out of these, CN and CO are strong field ligands and Cl is a weak field ligand. The electronic configuration of Ni is: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^8}\] . Hence, the valence shell configuration is:
In the case of strong field ligands, they will shift the electrons to higher energy positions to form completely filled orbitals. Hence, it could look like:
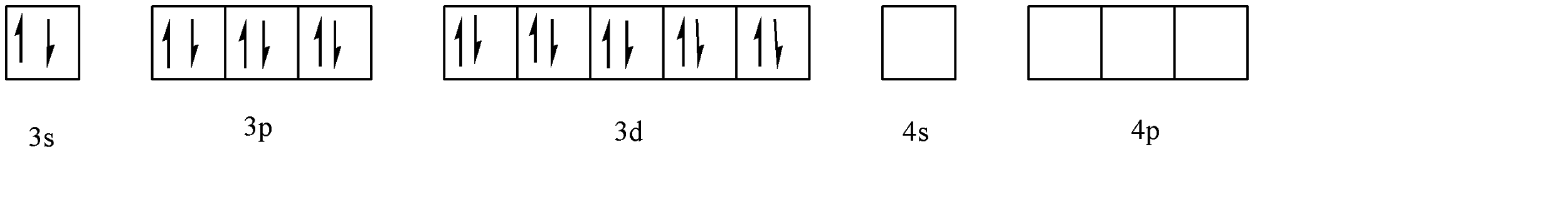
Hence the resultant hybridization is \[s{p^3}\]
Whereas in the case of weak field ligand, the shift of electrons to fit 4 substituents looks as follows:
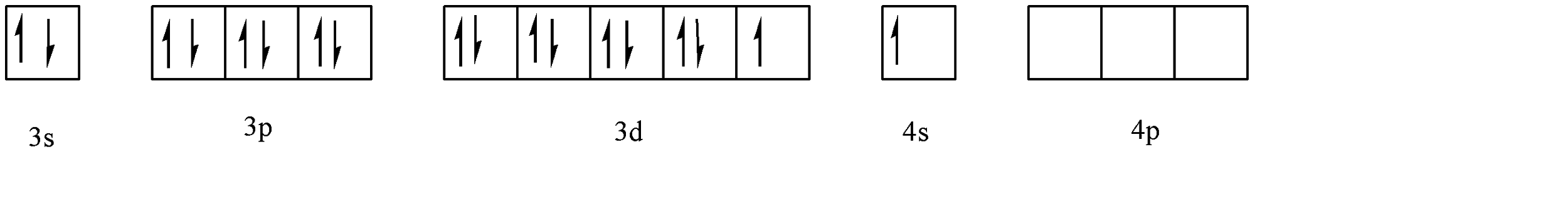
Hence, the hybridization for weak field ligands is \[ds{p^2}\]
Hence, among \[[Ni{(CO)_4}]\] , \[{[Ni{(CN)_4}]^{2 - }}\] , \[{[NiC{l_4}]^{2 - }}\] species, the hybridization states at the Ni atom are \[s{p^3}\] , \[s{p^3}\] , \[ds{p^2}\]
Hence, Option D is the correct option.
Note: There is a repulsion between the d orbitals of the central metal atom and the d orbitals of the ligands because of their similar electronic signature. Because of this, d electrons closer to the ligands will have a higher energy than those further away, which results in the d orbitals splitting in energy. The splitting of the d-orbitals into different energy levels in transition metal complexes has important consequences for their stability, reactivity, and magnetic properties.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Strong field ligands cause a large splitting in the given chemical species. This basically means strong field ligands exert strong ligand electrical fields. This property of strong field ligands makes them capable of forming low spin complexes. This means that strong field ligands disobey Hund’ multiplicity rule and cause pairing of electrons even before the entire subshell is filled with electrons of a specific spin character. On the other hand, weak field ligands obey Hund’s rule.
Moving back to the question, we can observe that the ligands attached to the Nickel atom are (CO), (CN) and (Cl). Out of these, CN and CO are strong field ligands and Cl is a weak field ligand. The electronic configuration of Ni is: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^8}\] . Hence, the valence shell configuration is:

In the case of strong field ligands, they will shift the electrons to higher energy positions to form completely filled orbitals. Hence, it could look like:

Hence the resultant hybridization is \[s{p^3}\]
Whereas in the case of weak field ligand, the shift of electrons to fit 4 substituents looks as follows:

Hence, the hybridization for weak field ligands is \[ds{p^2}\]
Hence, among \[[Ni{(CO)_4}]\] , \[{[Ni{(CN)_4}]^{2 - }}\] , \[{[NiC{l_4}]^{2 - }}\] species, the hybridization states at the Ni atom are \[s{p^3}\] , \[s{p^3}\] , \[ds{p^2}\]
Hence, Option D is the correct option.
Note: There is a repulsion between the d orbitals of the central metal atom and the d orbitals of the ligands because of their similar electronic signature. Because of this, d electrons closer to the ligands will have a higher energy than those further away, which results in the d orbitals splitting in energy. The splitting of the d-orbitals into different energy levels in transition metal complexes has important consequences for their stability, reactivity, and magnetic properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




