
Among ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ and ${\text{MeCN}}$ (${\text{Me}} = $methyl group) the electronegativity of ${\text{N}}$ is in the order of:
A) ${\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$
B) ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}} > {\text{MeCN}}$
C) ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}} > {\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$
D) Electronegativity is the same in all.
Answer
560.7k+ views
Hint: Electronegativity of an atom depends on its state of hybridisation. Determine the hybridisation of nitrogen i.e. ${\text{N}}$ in each of the given compounds. From the hybridisation, calculate the percent s-character and then decide the most electronegative carbon atom.
Complete step-by-step answer:
We are given three compounds ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ and ${\text{MeCN}}$ (${\text{Me}} = $methyl group). Let us calculate the percent s-character for nitrogen atom in each of the given compounds.
Consider the compound ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$:
The structure of the compound ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is as follows:
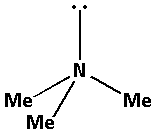
The nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ forms three bond pairs and also there is one lone pair of electron. Thus, ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ has a total four bond pairs. Thus, the nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is $s{p^3}$ hybridised.
Percentage s-character in $s{p^3}$ hybridised orbital $ = \dfrac{1}{4} = 0.25 \times 100\% = 25\% $.
Thus, the percentage s-character of the nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is $25\% $.
Consider the compound ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$:
The structure of the compound ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is as follows:
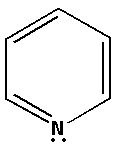
The nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ forms two bond pairs and also there is one lone pair of electron. Thus, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ has total three bond pairs. Thus, the nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is $s{p^2}$ hybridised.
Percentage s-character in $s{p^2}$ hybridised orbital $ = \dfrac{1}{3} = 0.33 \times 100\% = 33\% $.
Thus, the percentage s-character of the nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is $33\% $.
Consider the compound ${\text{MeCN}}$:
The structure of the compound ${\text{MeCN}}$ is as follows:
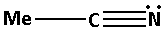
The nitrogen atom in ${\text{MeCN}}$ forms one bond pair and also there is one lone pair of electrons. Thus, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ has total two bond pairs. Thus, the nitrogen atom in ${\text{MeCN}}$ is sp hybridised.
Percentage s-character in sp hybridised orbital $ = \dfrac{1}{2} = 0.5 \times 100\% = 50\% $.
Thus, the percentage s-character of the nitrogen atom in ${\text{MeCN}}$ is $50\% $.
We know that more the s-character, more is the electronegativity of the nitrogen atom.
Thus, the electronegativity of ${\text{N}}$ is in the order of ${\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$.
Thus, the correct option is (A) ${\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$.
Note: Remember that more the s-character, more is the electronegativity of the nitrogen atom. Thus, the sp hybridised nitrogen atom is most electronegative than the $s{p^2}$ hybridised nitrogen atom which is more electronegative than the $s{p^3}$ hybridised nitrogen atom
Complete step-by-step answer:
We are given three compounds ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ and ${\text{MeCN}}$ (${\text{Me}} = $methyl group). Let us calculate the percent s-character for nitrogen atom in each of the given compounds.
Consider the compound ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$:
The structure of the compound ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is as follows:

The nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ forms three bond pairs and also there is one lone pair of electron. Thus, ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ has a total four bond pairs. Thus, the nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is $s{p^3}$ hybridised.
Percentage s-character in $s{p^3}$ hybridised orbital $ = \dfrac{1}{4} = 0.25 \times 100\% = 25\% $.
Thus, the percentage s-character of the nitrogen atom in ${\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$ is $25\% $.
Consider the compound ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$:
The structure of the compound ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is as follows:

The nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ forms two bond pairs and also there is one lone pair of electron. Thus, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ has total three bond pairs. Thus, the nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is $s{p^2}$ hybridised.
Percentage s-character in $s{p^2}$ hybridised orbital $ = \dfrac{1}{3} = 0.33 \times 100\% = 33\% $.
Thus, the percentage s-character of the nitrogen atom in ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ is $33\% $.
Consider the compound ${\text{MeCN}}$:
The structure of the compound ${\text{MeCN}}$ is as follows:
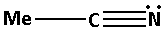
The nitrogen atom in ${\text{MeCN}}$ forms one bond pair and also there is one lone pair of electrons. Thus, ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}}$ has total two bond pairs. Thus, the nitrogen atom in ${\text{MeCN}}$ is sp hybridised.
Percentage s-character in sp hybridised orbital $ = \dfrac{1}{2} = 0.5 \times 100\% = 50\% $.
Thus, the percentage s-character of the nitrogen atom in ${\text{MeCN}}$ is $50\% $.
We know that more the s-character, more is the electronegativity of the nitrogen atom.
Thus, the electronegativity of ${\text{N}}$ is in the order of ${\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$.
Thus, the correct option is (A) ${\text{MeCN}} > {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{\text{N}} > {\text{M}}{{\text{e}}_{\text{3}}}{\text{N}}$.
Note: Remember that more the s-character, more is the electronegativity of the nitrogen atom. Thus, the sp hybridised nitrogen atom is most electronegative than the $s{p^2}$ hybridised nitrogen atom which is more electronegative than the $s{p^3}$ hybridised nitrogen atom
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




