
Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] the one with highest boiling point is:
A ) \[{{\text{H}}_2}{\text{O}}\] because of hydrogen bonding interactions.
B ) \[{{\text{H}}_2}{\text{Te}}\] because of its higher molecular weight.
C ) \[{{\text{H}}_2}{\text{S}}\] because of hydrogen bonding interactions.
D ) \[{{\text{H}}_2}{\text{Se}}\] because of its lower molecular weight.
Answer
584.4k+ views
Hint: A hydrogen bond is formed when hydrogen atom is attached to electronegative nitrogen, oxygen or fluorine atom. Due to formation of hydrogen bonds, molecules associated and more energy are needed to break these associations. This results in higher boiling. With increase in the molecular weight, the boiling point also increases. But the effect of hydrogen bonding is more pronounced than the effect of increase in the molecular weight.
Complete answer:
Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}}, {{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] the one that can form intermolecular hydrogen bonds is \[{{\text{H}}_2}{\text{O}}\]. This is because in \[{{\text{H}}_2}{\text{O}}\] molecules, a hydrogen atom is attached to an electronegative oxygen atom.
The hydrogen bonding in water molecules is as shown below:
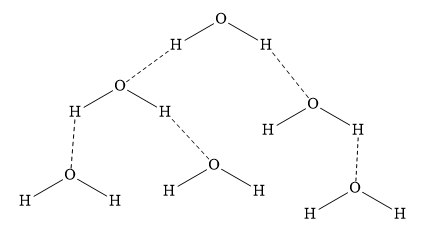
In the above diagram, the dotted line represents intermolecular hydrogen bonds.
\[{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] cannot form intermolecular hydrogen bonds as in these compounds, hydrogen atom is not attached to electronegative nitrogen, oxygen or fluorine atom.
Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] the one with highest boiling point is \[{{\text{H}}_2}{\text{O}}\].
Hence, the correct answer is the option A ).
Additional Information: In the oxygen family, the atomic mass increases in the order oxygen < sulfur < selenium < tellurium. On moving down the group the atomic mass increases. With increase in the atomic mass of the element, the molecular weight of its hydride also increases.
With increase in the molecular weight, the boiling point should also increase. But the effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight.
Note: Do not confuse between option A) and B) because of higher molecular weight of \[{{\text{H}}_2}{\text{Te}}\]. The effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight. Based on the combined effects of hydrogen bonding and the increase in the molecular weight, you can conclude that among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}}, {{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] , hydrogen sulfide \[{{\text{H}}_2}{\text{S}}\] has lowest boiling point as it has lowest molecular weight among \[{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] and it cannot form hydrogen bonds.
Complete answer:
Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}}, {{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] the one that can form intermolecular hydrogen bonds is \[{{\text{H}}_2}{\text{O}}\]. This is because in \[{{\text{H}}_2}{\text{O}}\] molecules, a hydrogen atom is attached to an electronegative oxygen atom.
The hydrogen bonding in water molecules is as shown below:

In the above diagram, the dotted line represents intermolecular hydrogen bonds.
\[{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] cannot form intermolecular hydrogen bonds as in these compounds, hydrogen atom is not attached to electronegative nitrogen, oxygen or fluorine atom.
Among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] the one with highest boiling point is \[{{\text{H}}_2}{\text{O}}\].
Hence, the correct answer is the option A ).
Additional Information: In the oxygen family, the atomic mass increases in the order oxygen < sulfur < selenium < tellurium. On moving down the group the atomic mass increases. With increase in the atomic mass of the element, the molecular weight of its hydride also increases.
With increase in the molecular weight, the boiling point should also increase. But the effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight.
Note: Do not confuse between option A) and B) because of higher molecular weight of \[{{\text{H}}_2}{\text{Te}}\]. The effect of hydrogen bonding on the boiling point outweighs the effect of increase in the molecular weight. Based on the combined effects of hydrogen bonding and the increase in the molecular weight, you can conclude that among \[{{\text{H}}_2}{\text{O, }}{{\text{H}}_2}{\text{S}}, {{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] , hydrogen sulfide \[{{\text{H}}_2}{\text{S}}\] has lowest boiling point as it has lowest molecular weight among \[{{\text{H}}_2}{\text{S}},{{\text{H}}_2}{\text{Se}}\] & \[{{\text{H}}_2}{\text{Te}}\] and it cannot form hydrogen bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




