
Ammonia is basic in nature hence it turns:
A.Red litmus to blue
B.Blue litmus to red
C.White litmus to red
D.White litmus to blue
Answer
566.7k+ views
Hint: Ammonia turns red litmus to blue so it is basic in nature. Ammonia is a compound of nitrogen and hydrogen with the chemical formula \[N{H_3}\]. It consists of one molecule of nitrogen and three molecules of hydrogen. A stable binary hydride and Pnictogen hydride nitrogen has a total of five electrons in the outer shell. It belongs to the \[15\] group of p-blocks. All basic nature elements turn the litmus paper red into blue due to the presence of lone pairs.
Complete step by step answer:
Nitrogen has a total of five electrons in the outermost shell. Nitrogen shares it’s three electrons with hydrogen to complete the octet so there is one lone pair in the \[N{H_3}\] molecule.
It acts as a Lewis base, so it can give an electron pair to two electrons deficient molecules. So due to the presence of one lone pair, it is basic in nature.
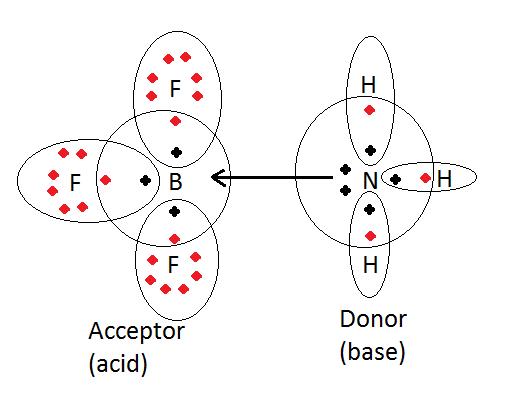
Lewis concept of acid and base for \[N{H_3}\]-
An acid is a substance (molecule or ion) which can accept a pair of electrons to form a coordinate bond. Here acid is an electron acceptor.
A base is a substance (molecule or ion) which can donate an electron pair to form a coordinate bond. Here the base is an electron donor.
According to Lewis concept, neutral molecules such as \[N{H_3}\] Having one or more lone pairs of electrons can act as Lewis bases.
\[B{F_3}(acid) \leftarrow N{H_3}(base)\]
Note:
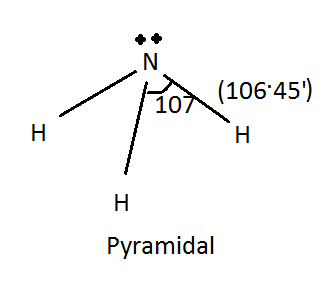
The \[N{H_3}\] has pyramidal structure and has \[s{p^3}\] hybridization. And the bond angle in \[N{H_3}\] molecule is \[ \sim {107^ \circ }\]. It can change the colour of litmus paper due to the above described reason as it is a Lewis base on the basis of the lone pair concept.
Complete step by step answer:
Nitrogen has a total of five electrons in the outermost shell. Nitrogen shares it’s three electrons with hydrogen to complete the octet so there is one lone pair in the \[N{H_3}\] molecule.
It acts as a Lewis base, so it can give an electron pair to two electrons deficient molecules. So due to the presence of one lone pair, it is basic in nature.
Lewis concept of acid and base for \[N{H_3}\]-
An acid is a substance (molecule or ion) which can accept a pair of electrons to form a coordinate bond. Here acid is an electron acceptor.
A base is a substance (molecule or ion) which can donate an electron pair to form a coordinate bond. Here the base is an electron donor.
According to Lewis concept, neutral molecules such as \[N{H_3}\] Having one or more lone pairs of electrons can act as Lewis bases.
\[B{F_3}(acid) \leftarrow N{H_3}(base)\]

Note:
The \[N{H_3}\] has pyramidal structure and has \[s{p^3}\] hybridization. And the bond angle in \[N{H_3}\] molecule is \[ \sim {107^ \circ }\]. It can change the colour of litmus paper due to the above described reason as it is a Lewis base on the basis of the lone pair concept.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




