
Ammonia is a good complexing agent. Explain with an example.
Answer
569.1k+ views
Hint: We know that any substance that is capable of forming a complex compound with another material in a solution is known as a complexing agent. Ammonia generally forms complexes with metals ions.
Complete answer:
We know that the molecular formula for ammonia is ${\text{N}}{{\text{H}}_{\text{3}}}$. Ammonia is also known as nitrogen trihydride or azane. In the structure of ammonia, three hydrogen atoms are connected to the central nitrogen atom.
The structure of ammonia is as follows:
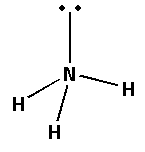
From the structure of ammonia, we can see that the shape of the ammonia molecule is trigonal pyramidal. The molecule of ammonia constantly undergoes inversion motion.
We can see that there is a lone pair of electrons on the nitrogen atom of ammonia. As there is a lone pair of electrons the ammonia molecule has a good tendency to donate electrons. As there is a lone pair of electrons on the nitrogen atom, the ammonia molecule acts as a good Lewis base.Thus, as ammonia has a good tendency to donate electrons, it has a good ability to form stable complexes.
Thus, ammonia is a good complexing agent.
The example of ammonia as a good complexing agent is as follows:
i) When we test a sample for the presence of chloride ions we use silver nitrate solution. The chloride ions react with silver nitrate and form a white precipitate of silver chloride. The reaction is as follows:
${\text{AgN}}{{\text{O}}_3} + {\text{C}}{{\text{l}}^ - } \to {\text{AgCl}} + {\text{NO}}_3^ - $
ii) When the precipitate of silver chloride is added to the solution of ammonia, the precipitate dissolves in the solution. This is because ammonia forms a complex with silver metal and chloride ions are set free. The reaction is as follows:
${\text{AgCl}} + 2{\text{N}}{{\text{H}}_{\text{3}}} \to {\left[ {{\text{Ag}}{{\left( {{\text{N}}{{\text{H}}_3}} \right)}_2}} \right]^ + } + {\text{C}}{{\text{l}}^ - }$
iii) The complex formed ${\left[ {{\text{Ag}}{{\left( {{\text{N}}{{\text{H}}_3}} \right)}_2}} \right]^ + }$ is the silver-ammonia complex.
Note:Almost all the metals bind to ammonia to form complexes. Ammonia thus, is known as a ligand. Most common complexes of ammonia are with metals like chromium, cobalt, nickel, copper and the metals of platinum group.
Complete answer:
We know that the molecular formula for ammonia is ${\text{N}}{{\text{H}}_{\text{3}}}$. Ammonia is also known as nitrogen trihydride or azane. In the structure of ammonia, three hydrogen atoms are connected to the central nitrogen atom.
The structure of ammonia is as follows:

From the structure of ammonia, we can see that the shape of the ammonia molecule is trigonal pyramidal. The molecule of ammonia constantly undergoes inversion motion.
We can see that there is a lone pair of electrons on the nitrogen atom of ammonia. As there is a lone pair of electrons the ammonia molecule has a good tendency to donate electrons. As there is a lone pair of electrons on the nitrogen atom, the ammonia molecule acts as a good Lewis base.Thus, as ammonia has a good tendency to donate electrons, it has a good ability to form stable complexes.
Thus, ammonia is a good complexing agent.
The example of ammonia as a good complexing agent is as follows:
i) When we test a sample for the presence of chloride ions we use silver nitrate solution. The chloride ions react with silver nitrate and form a white precipitate of silver chloride. The reaction is as follows:
${\text{AgN}}{{\text{O}}_3} + {\text{C}}{{\text{l}}^ - } \to {\text{AgCl}} + {\text{NO}}_3^ - $
ii) When the precipitate of silver chloride is added to the solution of ammonia, the precipitate dissolves in the solution. This is because ammonia forms a complex with silver metal and chloride ions are set free. The reaction is as follows:
${\text{AgCl}} + 2{\text{N}}{{\text{H}}_{\text{3}}} \to {\left[ {{\text{Ag}}{{\left( {{\text{N}}{{\text{H}}_3}} \right)}_2}} \right]^ + } + {\text{C}}{{\text{l}}^ - }$
iii) The complex formed ${\left[ {{\text{Ag}}{{\left( {{\text{N}}{{\text{H}}_3}} \right)}_2}} \right]^ + }$ is the silver-ammonia complex.
Note:Almost all the metals bind to ammonia to form complexes. Ammonia thus, is known as a ligand. Most common complexes of ammonia are with metals like chromium, cobalt, nickel, copper and the metals of platinum group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




