
Although geometries of \[N{{H}_{3}}\] and \[{{H}_{2}}O\] molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Explain.
Answer
588.9k+ views
Hint: Whenever we are going to know about structure and bond angles in the molecules first we have to see how many bond pairs and lone pairs are present in the structure of the molecules. The presence of lone pairs of electrons is the reason behind the change in the bond angle in the molecules.
Complete step by step answer:
The structure of Ammonia is as follows.
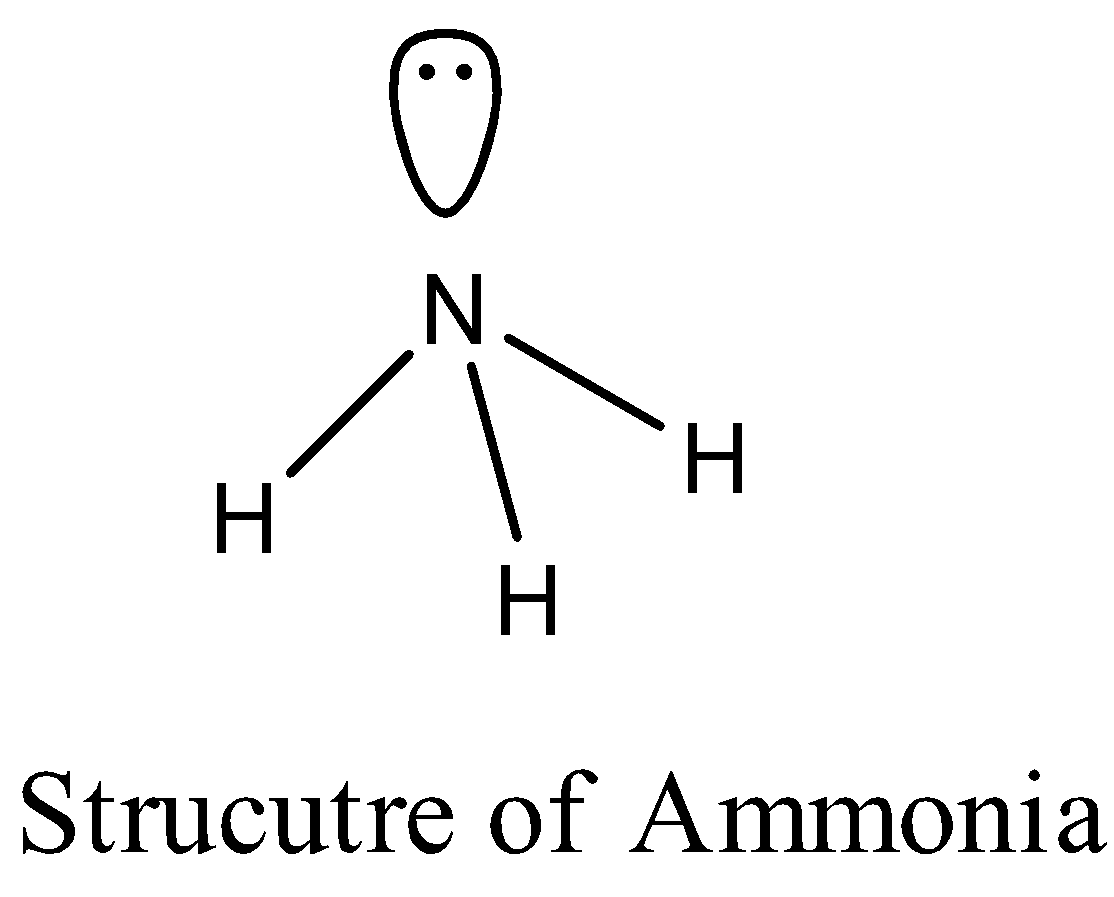
The structure of water is as follows.

In the structure of ammonia we can see clearly that there are three bond pairs and one lone pair of electrons.
In the structure of water we can see that there are two bond pairs and two lone pairs of electrons.
Due to the presence of one lone pair of electrons in the structure of ammonia the bond angle is decreased from \[{{109}^{o}}\] to \[{{107}^{o}}\].
Due to the presence of two lone pair of electrons in structure of water molecule the bond angle is decreased from \[{{107}^{o}}\] to \[{{105.4}^{o}}\].
In ammonia only one lone pair of electrons are present, but in water molecules there are two lone pairs of electrons.
Due to the presence of two lone pairs of electrons in the structure of the water molecule there is a high repulsion between two bond pairs and two lone pairs when compared to one lone pair and three bond pair repulsions in ammonia.
Then the repulsions between two bond pairs and two lone pairs in water molecules are greater than two bond pairs and two lone pairs that have ammonia. So, the bond angle in water is less than that of bond angle in ammonia.
Note: The repulsion between bond pairs and lone pairs are as follows.
Bond pair - bond pair < bond pair – lone pair < lone pair- lone pair.
Means lone pair-lone pair repulsions are high when compared to bond pair-lone pair repulsions.
Bond pair-lone pair repulsions are high when compared to bond pair-bond pair repulsions.
Complete step by step answer:
The structure of Ammonia is as follows.

The structure of water is as follows.

In the structure of ammonia we can see clearly that there are three bond pairs and one lone pair of electrons.
In the structure of water we can see that there are two bond pairs and two lone pairs of electrons.
Due to the presence of one lone pair of electrons in the structure of ammonia the bond angle is decreased from \[{{109}^{o}}\] to \[{{107}^{o}}\].
Due to the presence of two lone pair of electrons in structure of water molecule the bond angle is decreased from \[{{107}^{o}}\] to \[{{105.4}^{o}}\].
In ammonia only one lone pair of electrons are present, but in water molecules there are two lone pairs of electrons.
Due to the presence of two lone pairs of electrons in the structure of the water molecule there is a high repulsion between two bond pairs and two lone pairs when compared to one lone pair and three bond pair repulsions in ammonia.
Then the repulsions between two bond pairs and two lone pairs in water molecules are greater than two bond pairs and two lone pairs that have ammonia. So, the bond angle in water is less than that of bond angle in ammonia.
Note: The repulsion between bond pairs and lone pairs are as follows.
Bond pair - bond pair < bond pair – lone pair < lone pair- lone pair.
Means lone pair-lone pair repulsions are high when compared to bond pair-lone pair repulsions.
Bond pair-lone pair repulsions are high when compared to bond pair-bond pair repulsions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




