
Allyl Bromide is:
A.
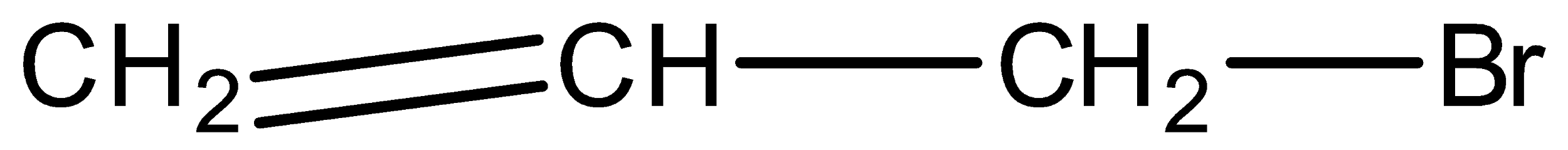
B.
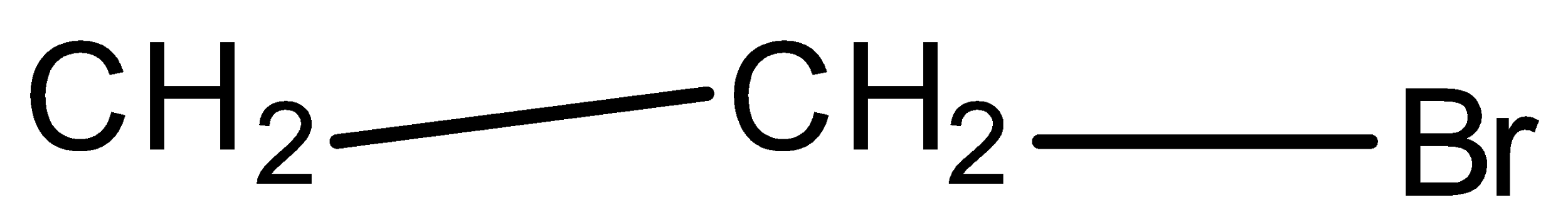
C.
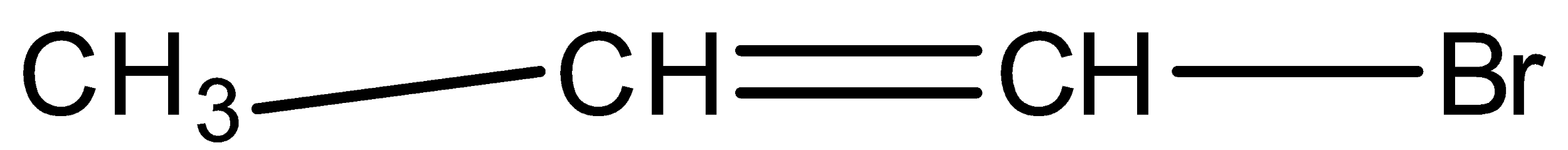
D.
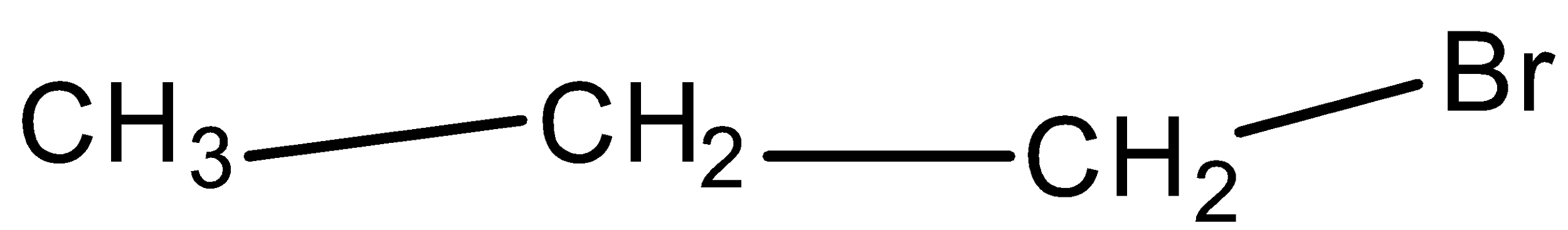
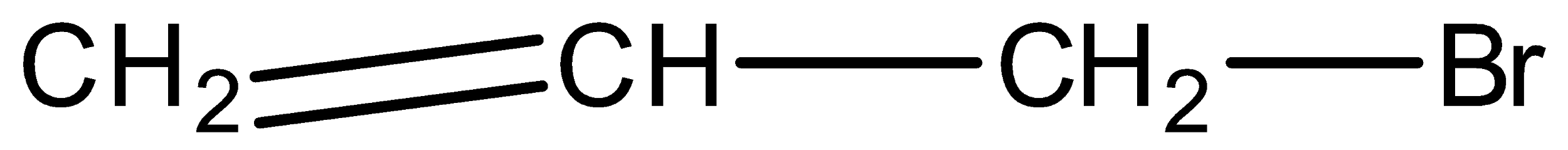
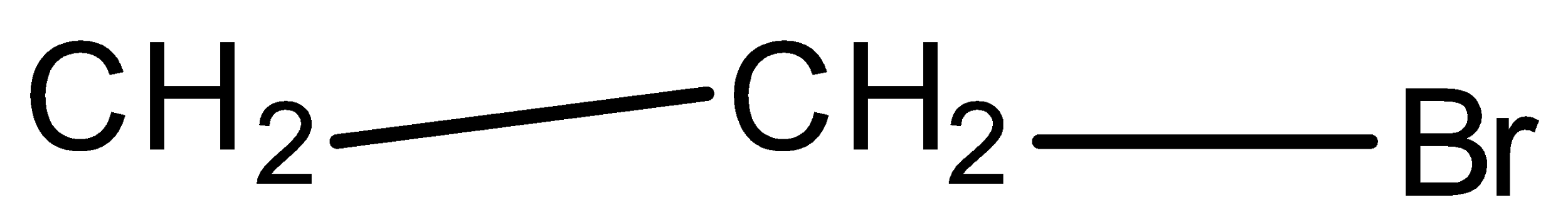
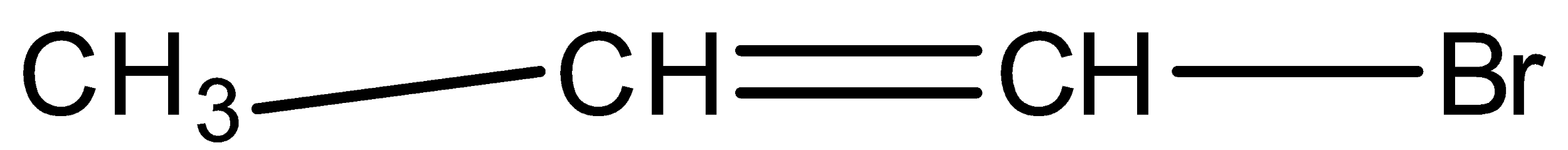
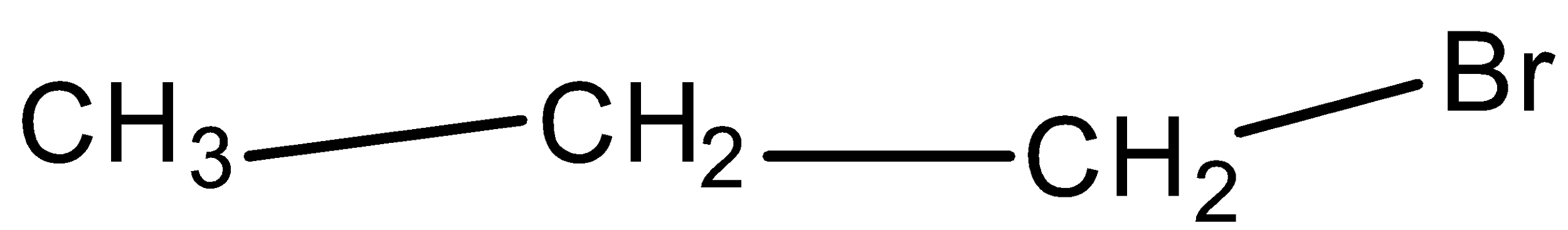
Answer
577.5k+ views
Hint: Allyl bromide can be defined as the bromine attached to the carbon attached to the double bonded carbon atom . Allyl bromide is an alkyl halide where bromine can be replaced by any halogens like fluorine ,chlorine ,bromine, iodine.
Complete answer:
In an organic compound like \[{{C}_{3}}{{H}_{5}}Br\] if the bromine is attached to the carbon which is attached with the double bonded carbon then it is classified as allyl bromide or allyl halide.
Allyl bromide is an alkylating agent which is generally used in the synthesis of polymers, pharmaceuticals and many other organic compounds. Physical characteristics of allyl bromide consists of a colourless liquid with intense, acrid and persistent smell. Density of allyl bromide is generally 1.4 gram per centimetre cube. Molar mass of allyl bromide is 120.99 gram per mole. Boiling point of allyl bromide is 71 degree centigrade.
It is generally produced commercially from allyl alcohol. Alternatively allyl chloride does react with the hydrogen bromide in the presence of copper bromide. This compound is mainly used as an electrophilic alkylating agent. Allyl zinc bromide can be produced by treating this compound with elemental zinc.
So according to the definitions and theory mentioned above the correct answer from the above options is option number ( A).
As only in option A the bromine is attached to the carbon which is again attached to the double bonded carbon atom.
So our suitable answer is option A.
Note:
If the bromine or halogen are attached directly to the double bonded carbon atom then it is known as vinyl bromide or vinyl halide. Allyl bromide is used in the synthesis of polymers, medicines and preparation of other compounds.
Complete answer:
In an organic compound like \[{{C}_{3}}{{H}_{5}}Br\] if the bromine is attached to the carbon which is attached with the double bonded carbon then it is classified as allyl bromide or allyl halide.
Allyl bromide is an alkylating agent which is generally used in the synthesis of polymers, pharmaceuticals and many other organic compounds. Physical characteristics of allyl bromide consists of a colourless liquid with intense, acrid and persistent smell. Density of allyl bromide is generally 1.4 gram per centimetre cube. Molar mass of allyl bromide is 120.99 gram per mole. Boiling point of allyl bromide is 71 degree centigrade.
It is generally produced commercially from allyl alcohol. Alternatively allyl chloride does react with the hydrogen bromide in the presence of copper bromide. This compound is mainly used as an electrophilic alkylating agent. Allyl zinc bromide can be produced by treating this compound with elemental zinc.
So according to the definitions and theory mentioned above the correct answer from the above options is option number ( A).
As only in option A the bromine is attached to the carbon which is again attached to the double bonded carbon atom.
So our suitable answer is option A.
Note:
If the bromine or halogen are attached directly to the double bonded carbon atom then it is known as vinyl bromide or vinyl halide. Allyl bromide is used in the synthesis of polymers, medicines and preparation of other compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




