
Allotropes of oxygen are
(a). $O_2$
(b). $O_3$
(c). Both A & B
(d). None
Answer
607.5k+ views
Hint: Remember the definition of allotrope. It has one or more forms of chemical elements that occur in the same physical state. It may display very different physical and chemical properties.
Complete step by step solution:
Allotropy is the property of some chemical elements to exist in two or more different forms, or allotropes, when found in nature. There are several allotropes of carbon.
Elements such as carbon, oxygen, phosphorus, tin and sulfur, display the property known as allotropy.
The different physical properties displayed by allotropes of an element are explained by the fact that the atoms are arranged into molecules or crystals in different ways.
Some allotropes of an element may be more chemically stable than others.
Oxygen's most common allotrope is diatomic oxygen or $O_2$, a reactive paramagnetic molecule and ozone, ${O_3}$, is another allotrope of oxygen. Ozone has a pungent odor, and its color is blue-black in its solid and liquid form. Ozone is used to deodorize air, purify water, and treat industrial wastes.
Lewis structure of ${O_2}$:
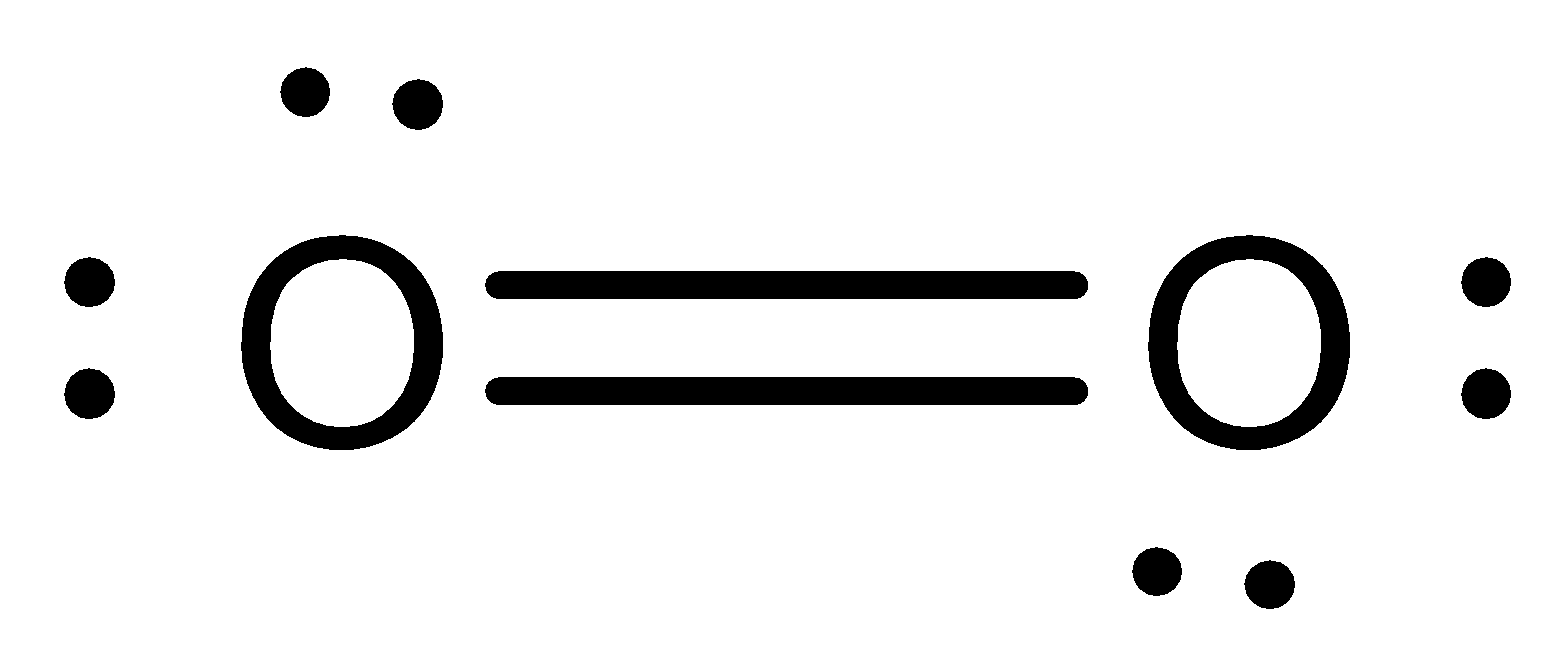
Lewis structure of $O_3$:
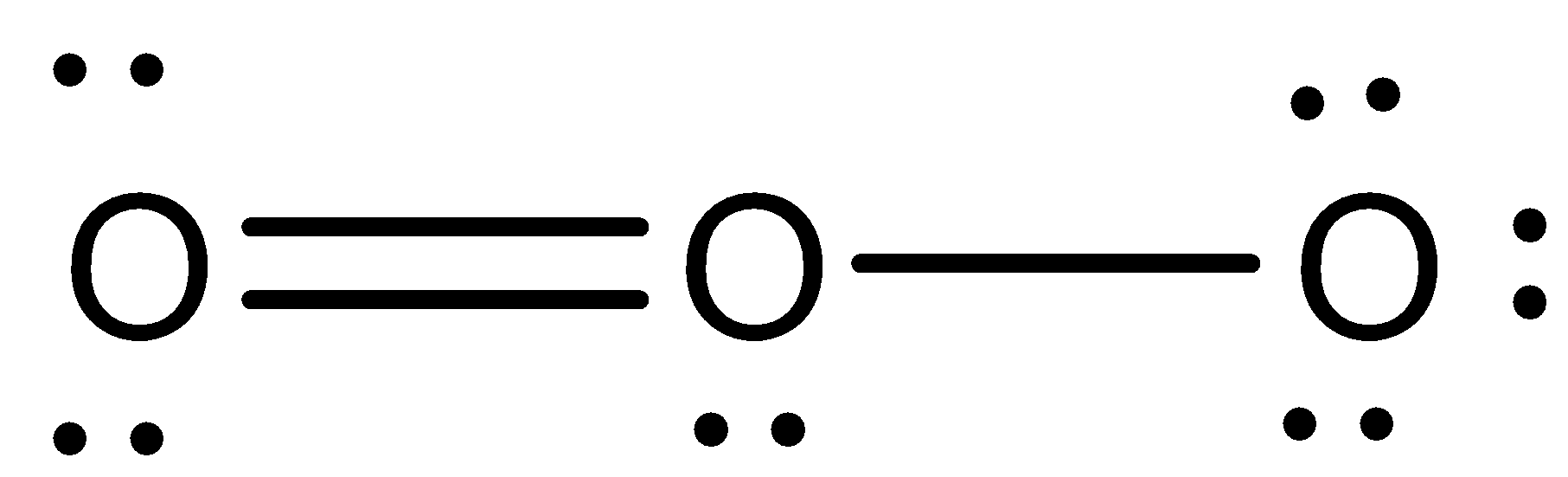
Some of the similarities of the allotropes are:
1. Gaseous
2. Decomposed by UV light
3. Low melting and boiling point
4. Contain covalent bonding
5. Formed with only oxygen atom
Hence, the correct option is (c) Both A & B.
Note: It should not be confused with the concept that allotropes have the same physical and chemical properties.
Complete step by step solution:
Allotropy is the property of some chemical elements to exist in two or more different forms, or allotropes, when found in nature. There are several allotropes of carbon.
Elements such as carbon, oxygen, phosphorus, tin and sulfur, display the property known as allotropy.
The different physical properties displayed by allotropes of an element are explained by the fact that the atoms are arranged into molecules or crystals in different ways.
Some allotropes of an element may be more chemically stable than others.
Oxygen's most common allotrope is diatomic oxygen or $O_2$, a reactive paramagnetic molecule and ozone, ${O_3}$, is another allotrope of oxygen. Ozone has a pungent odor, and its color is blue-black in its solid and liquid form. Ozone is used to deodorize air, purify water, and treat industrial wastes.
Lewis structure of ${O_2}$:
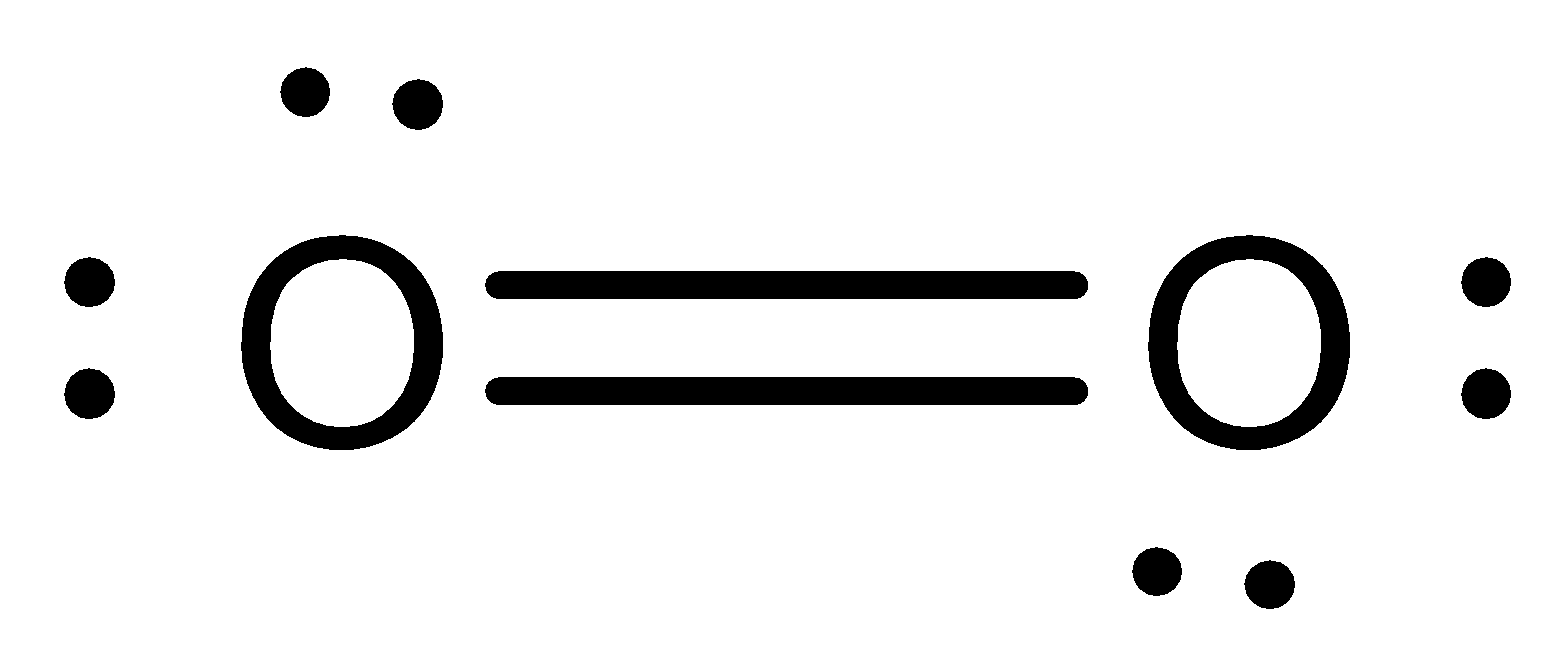
Lewis structure of $O_3$:
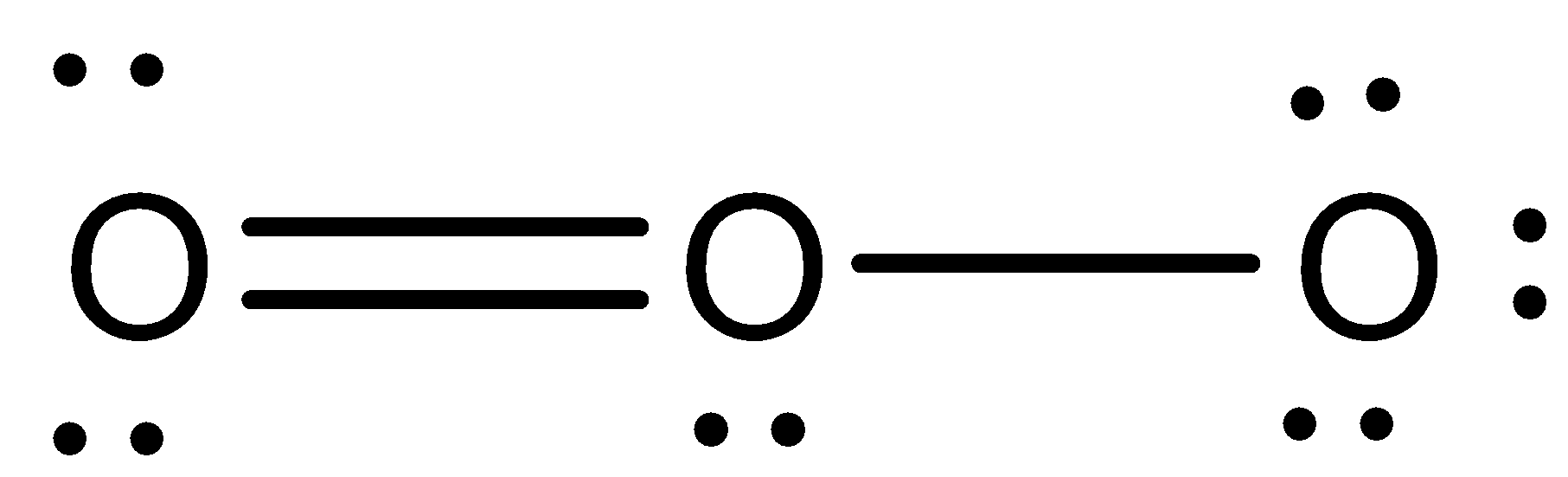
Some of the similarities of the allotropes are:
1. Gaseous
2. Decomposed by UV light
3. Low melting and boiling point
4. Contain covalent bonding
5. Formed with only oxygen atom
Hence, the correct option is (c) Both A & B.
Note: It should not be confused with the concept that allotropes have the same physical and chemical properties.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




