
All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are equivalent
A ) True
B ) False
Answer
582.3k+ views
Hint: Electron deficient compounds have coordinate covalent bonds in which number of electrons are lesser than that required for the formation of normal covalent bonds. When the electronegativity difference between two atoms is less than 1.7, coordinate covalent bonds are formed.
Complete answer:
Aluminum has three valence electrons whereas chlorine has seven valence electrons. One aluminum atom shares three electrons with three chlorine atoms to form three \[{\rm{Al}} - {\rm{Cl}}\] bonds.
The structure of aluminum chloride varies with change in temperature. It also changes with change in the physical state, solid, liquid or gas. In solid state, aluminum chloride has a cubic close packed layered structure. In a liquid and gaseous state, a dimer is formed. In the dimer, each aluminum atom has tetrahedral geometry. At high temperature, trigonal planar monomer is present.
The given statement is false. All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are not equivalent.
Some \[{\rm{Al}} - {\rm{Cl}}\] bonds are covalent whereas other \[{\rm{Al}} - {\rm{Cl}}\] bonds are coordinate.
Terminal \[{\rm{Al}} - {\rm{Cl}}\] bonds are normal covalent bonds. They are \[2c - 2e\] bonds.
The bonds containing \[{\rm{Al}} - {\rm{Cl}} - {\rm{Al}}\] bridges are \[3c - 2e\] bonds.
Write down the structure of \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] dimer as shown below.
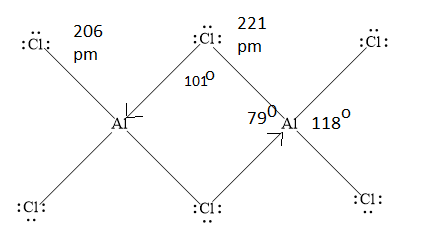
Hence, the option B ) is the correct option.
Note: \[{{\rm{B}}_2}{{\rm{F}}_6}\] is also an electron deficient compound and it involves halogen bridges. \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] is an electron deficient compound. The number of electrons in the valence shell is less than that required to complete the octet.
Complete answer:
Aluminum has three valence electrons whereas chlorine has seven valence electrons. One aluminum atom shares three electrons with three chlorine atoms to form three \[{\rm{Al}} - {\rm{Cl}}\] bonds.
The structure of aluminum chloride varies with change in temperature. It also changes with change in the physical state, solid, liquid or gas. In solid state, aluminum chloride has a cubic close packed layered structure. In a liquid and gaseous state, a dimer is formed. In the dimer, each aluminum atom has tetrahedral geometry. At high temperature, trigonal planar monomer is present.
The given statement is false. All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are not equivalent.
Some \[{\rm{Al}} - {\rm{Cl}}\] bonds are covalent whereas other \[{\rm{Al}} - {\rm{Cl}}\] bonds are coordinate.
Terminal \[{\rm{Al}} - {\rm{Cl}}\] bonds are normal covalent bonds. They are \[2c - 2e\] bonds.
The bonds containing \[{\rm{Al}} - {\rm{Cl}} - {\rm{Al}}\] bridges are \[3c - 2e\] bonds.
Write down the structure of \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] dimer as shown below.

Hence, the option B ) is the correct option.
Note: \[{{\rm{B}}_2}{{\rm{F}}_6}\] is also an electron deficient compound and it involves halogen bridges. \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] is an electron deficient compound. The number of electrons in the valence shell is less than that required to complete the octet.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




