
All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are equivalent
A ) True
B ) False
Answer
592.2k+ views
Hint: Electron deficient compounds have coordinate covalent bonds in which number of electrons are lesser than that required for the formation of normal covalent bonds. When the electronegativity difference between two atoms is less than 1.7, coordinate covalent bonds are formed.
Complete answer:
Aluminum has three valence electrons whereas chlorine has seven valence electrons. One aluminum atom shares three electrons with three chlorine atoms to form three \[{\rm{Al}} - {\rm{Cl}}\] bonds.
The structure of aluminum chloride varies with change in temperature. It also changes with change in the physical state, solid, liquid or gas. In solid state, aluminum chloride has a cubic close packed layered structure. In a liquid and gaseous state, a dimer is formed. In the dimer, each aluminum atom has tetrahedral geometry. At high temperature, trigonal planar monomer is present.
The given statement is false. All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are not equivalent.
Some \[{\rm{Al}} - {\rm{Cl}}\] bonds are covalent whereas other \[{\rm{Al}} - {\rm{Cl}}\] bonds are coordinate.
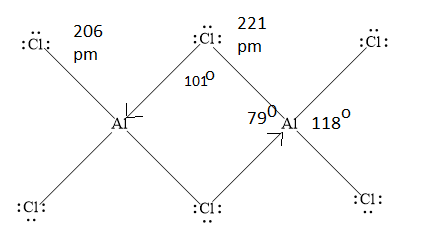
Terminal \[{\rm{Al}} - {\rm{Cl}}\] bonds are normal covalent bonds. They are \[2c - 2e\] bonds.
The bonds containing \[{\rm{Al}} - {\rm{Cl}} - {\rm{Al}}\] bridges are \[3c - 2e\] bonds.
Write down the structure of \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] dimer as shown below.
Hence, the option B ) is the correct option.
Note: \[{{\rm{B}}_2}{{\rm{F}}_6}\] is also an electron deficient compound and it involves halogen bridges. \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] is an electron deficient compound. The number of electrons in the valence shell is less than that required to complete the octet.
Complete answer:
Aluminum has three valence electrons whereas chlorine has seven valence electrons. One aluminum atom shares three electrons with three chlorine atoms to form three \[{\rm{Al}} - {\rm{Cl}}\] bonds.
The structure of aluminum chloride varies with change in temperature. It also changes with change in the physical state, solid, liquid or gas. In solid state, aluminum chloride has a cubic close packed layered structure. In a liquid and gaseous state, a dimer is formed. In the dimer, each aluminum atom has tetrahedral geometry. At high temperature, trigonal planar monomer is present.
The given statement is false. All the \[{\rm{Al}} - {\rm{Cl}}\] bonds in \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] are not equivalent.
Some \[{\rm{Al}} - {\rm{Cl}}\] bonds are covalent whereas other \[{\rm{Al}} - {\rm{Cl}}\] bonds are coordinate.
Terminal \[{\rm{Al}} - {\rm{Cl}}\] bonds are normal covalent bonds. They are \[2c - 2e\] bonds.
The bonds containing \[{\rm{Al}} - {\rm{Cl}} - {\rm{Al}}\] bridges are \[3c - 2e\] bonds.
Write down the structure of \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] dimer as shown below.

Hence, the option B ) is the correct option.
Note: \[{{\rm{B}}_2}{{\rm{F}}_6}\] is also an electron deficient compound and it involves halogen bridges. \[{\rm{A}}{{\rm{l}}_2}{\rm{C}}{{\rm{l}}_6}\] is an electron deficient compound. The number of electrons in the valence shell is less than that required to complete the octet.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




