
All the atoms are composed of subatomic parts which are known as charged particles?
(A). Electrons and Protons
(B). Protons and neutrons
(C). Electrons and neutrons
(D). Only neutrons
Answer
607.5k+ views
- Hint: The smallest particle of a chemical element that can exist is an atom or we can say that atoms are the basic units of matter and the defining structure of elements. Every solid, liquid, gas, and plasma are composed of neutral or ionized atoms, they are extremely small to see from naked eyes. Refer to the structure of an atom to get the answer of the given question.
Complete step-by-step solution -
As we know that an atom is the basic unit of any matter and it is a defining structure of elements.
A typical atom consists of three subatomic particles which are:
Electrons, protons and neutrons.
Refer the structure below for better understanding-
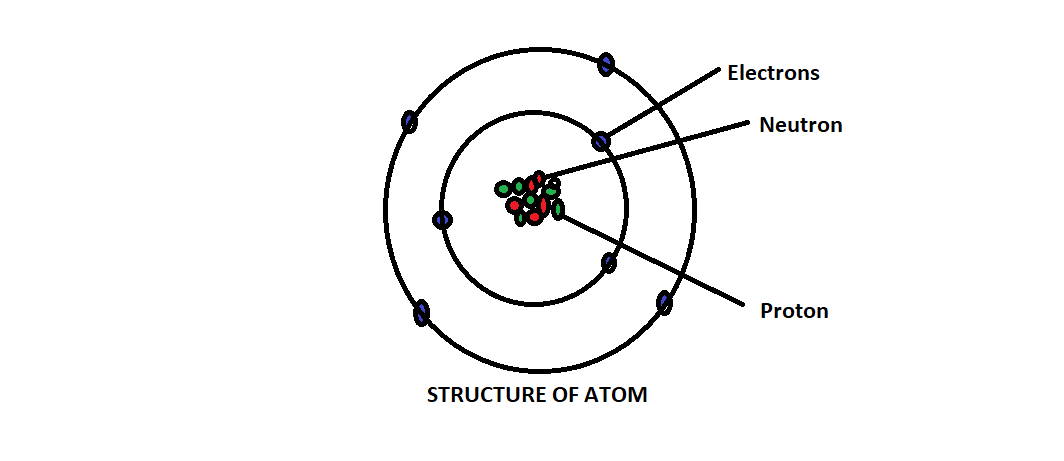
Protons and neutrons are heavier than electrons and they reside inside the nucleus at the centre of the atom.
Electrons are extremely lightweight and exist in clouds orbiting the nucleus.
Electrons have negative charge, protons have positive charge and neutrons have no charge, they are neutral.
This means that all the atoms are composed of subatomic parts which are known as charged particles are electrons and protons.
Hence, the correct option is A.
Note- While writing the answer to such types of questions, you should know the basic structure of an atom. As we know atoms consist of three subatomic particles, but among these three subatomic parts, the charged particles are only electrons and protons as neutrons are neutral. So, we found that the option A is correct and best suitable in regards to an atom.
Complete step-by-step solution -
As we know that an atom is the basic unit of any matter and it is a defining structure of elements.
A typical atom consists of three subatomic particles which are:
Electrons, protons and neutrons.
Refer the structure below for better understanding-

Protons and neutrons are heavier than electrons and they reside inside the nucleus at the centre of the atom.
Electrons are extremely lightweight and exist in clouds orbiting the nucleus.
Electrons have negative charge, protons have positive charge and neutrons have no charge, they are neutral.
This means that all the atoms are composed of subatomic parts which are known as charged particles are electrons and protons.
Hence, the correct option is A.
Note- While writing the answer to such types of questions, you should know the basic structure of an atom. As we know atoms consist of three subatomic particles, but among these three subatomic parts, the charged particles are only electrons and protons as neutrons are neutral. So, we found that the option A is correct and best suitable in regards to an atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




