
How many alkaline could you treat with $ {H_2} $ $ Pd/C $ in order to prepare methylcyclopentane?
Answer
531.3k+ views
Hint : $ {H_2} $ and $ Pd/C $ will reduce alkynes to alkanes it means two hydrogen atoms get added to it . Palladium catalyses the addition of hydrogen to multiple carbon-carbon bonds. This reaction will take place twice while reducing alkynes to alkanes.
Complete Step By Step Answer:
To prepare methylcyclopentane by using $ {H_2} $ , $ Pd/C $ the required alkaline are $ 4 - $ methylcyclopent $ - 1 - $ ene, $ 1 - $ methylcyclopent $ - 1 - $ ene, methylenecyclopentane and $ 3 - $ methylcyclopent $ - 1 - $ ene.
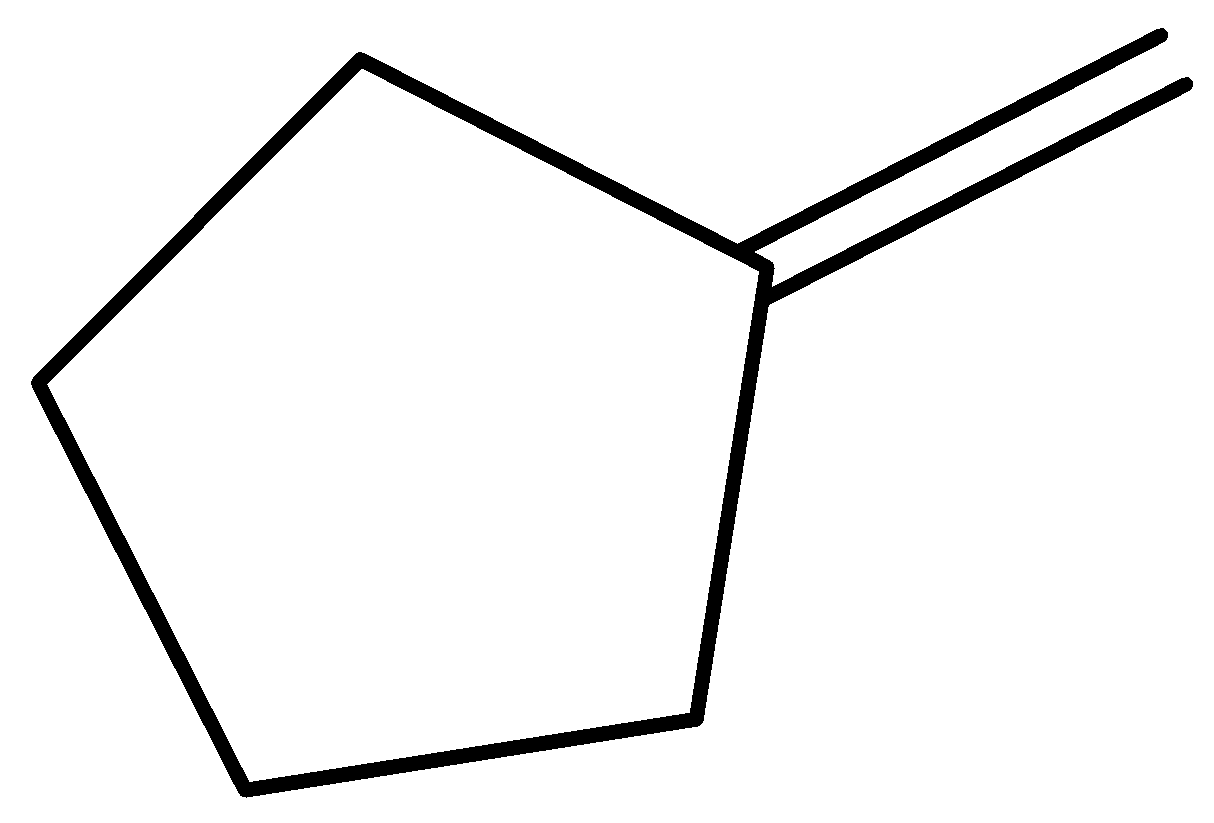
Methylenecyclopentane
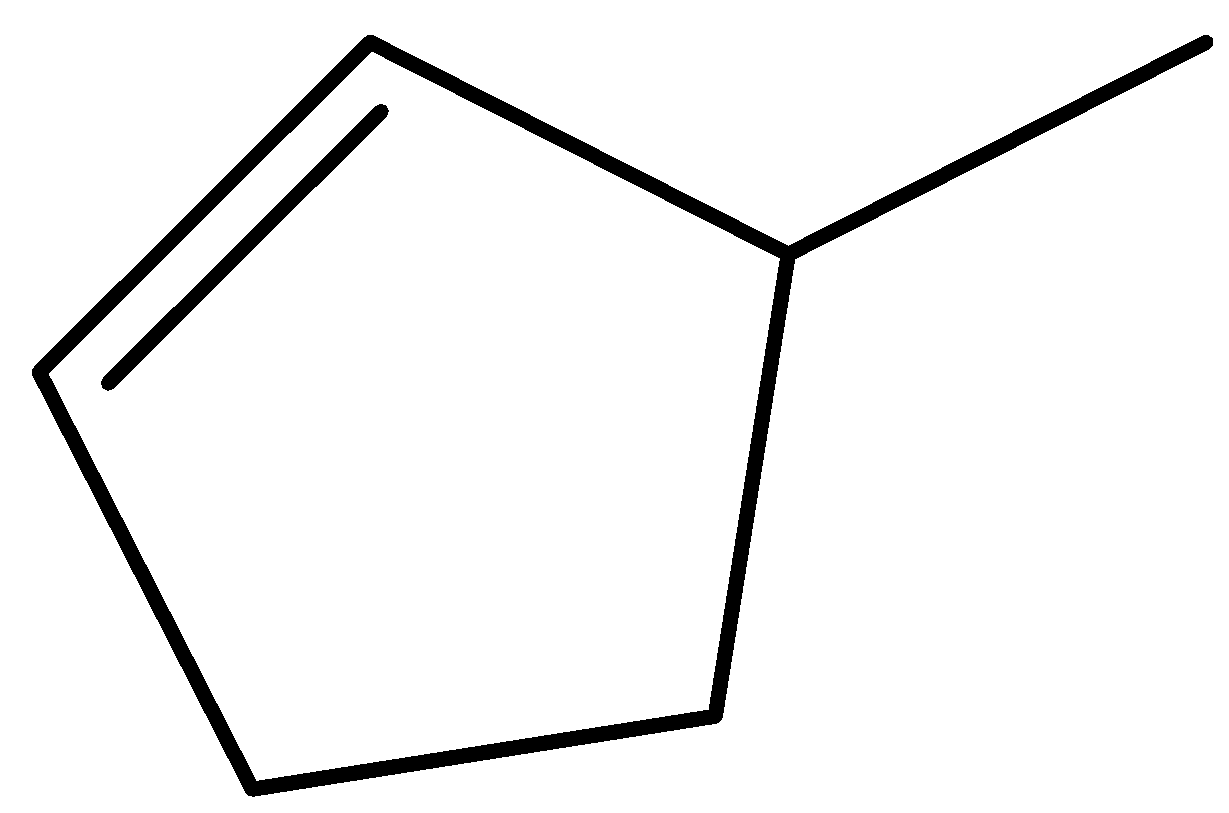
$ 3 - $ methylcyclopent $ - 1 - $ ene.
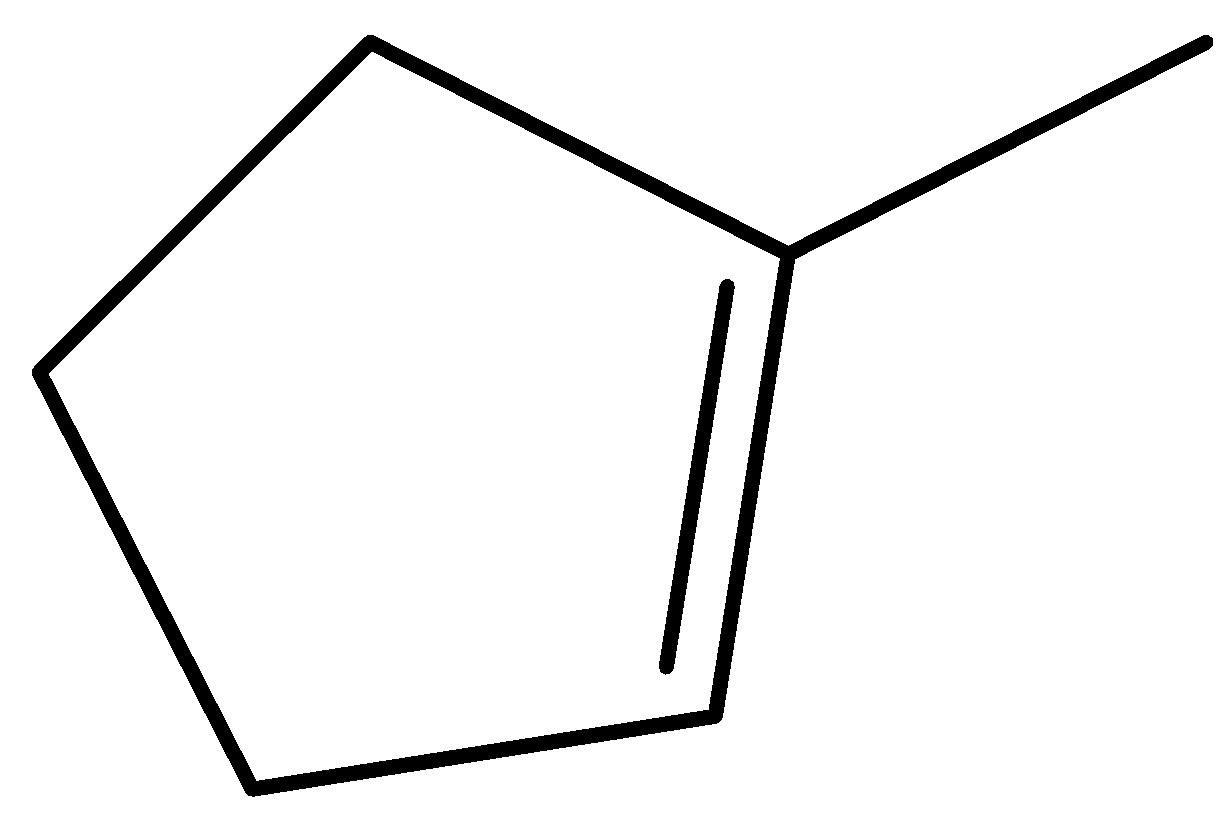
$ 1 - $ methylcyclopent $ - 1 - $ ene
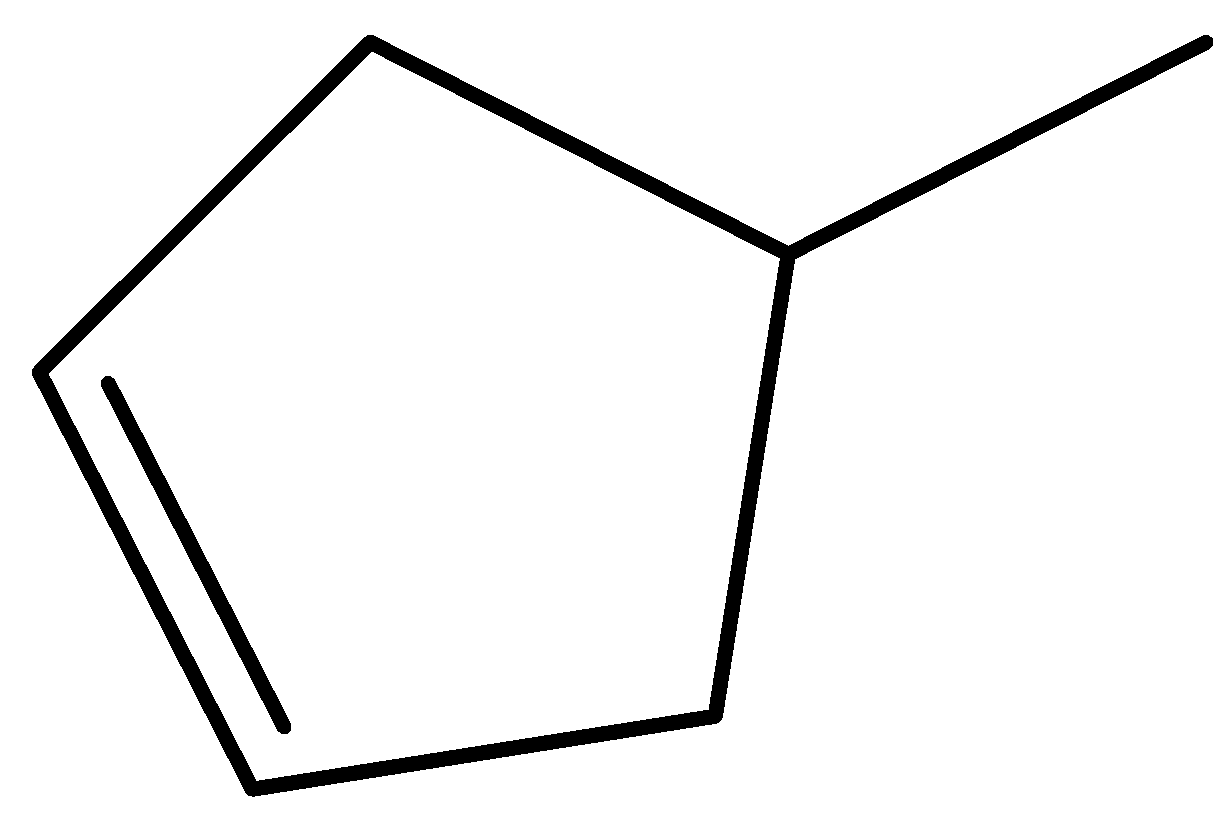
$ 4 - $ methylcyclopent $ - 1 - $ ene
Note :
This hydrogenation reaction catches fire very easily as it has flammable reagents and solvents as it includes palladium and carbon; they are highly flammable in nature and can ignite fire very easily. The presence of hydrogen gas adds risk of explosion.
Complete Step By Step Answer:
To prepare methylcyclopentane by using $ {H_2} $ , $ Pd/C $ the required alkaline are $ 4 - $ methylcyclopent $ - 1 - $ ene, $ 1 - $ methylcyclopent $ - 1 - $ ene, methylenecyclopentane and $ 3 - $ methylcyclopent $ - 1 - $ ene.

Methylenecyclopentane

$ 3 - $ methylcyclopent $ - 1 - $ ene.

$ 1 - $ methylcyclopent $ - 1 - $ ene

$ 4 - $ methylcyclopent $ - 1 - $ ene
Note :
This hydrogenation reaction catches fire very easily as it has flammable reagents and solvents as it includes palladium and carbon; they are highly flammable in nature and can ignite fire very easily. The presence of hydrogen gas adds risk of explosion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




