
$Al{F_3}$is soluble in $HF$only in presence of $KF$. It is due to the formation of:
A. $Al{H_3}$
B. $K\left[ {Al{F_3}H} \right]$
C. ${K_3}[ {Al{F_3}{H_3}}]$
D. ${K_3}[ {Al{F_6}}]$
Answer
584.7k+ views
Hint: $Al{F_3}$ is soluble only in presence of $HF$ only in presence of $KF$. It is due to formation of a complex ${K_3}\left[ {Al{F_6}} \right]$,
Complete step by step answer: Aluminium form fluoride complexes more easily than in the case of silicon. So, $Al{F_3}$ easily dissolves in a mixture of $\left( {HF + KF} \right)$.
$Al{F_3}$ is soluble in $HF$ due to the formation of ${K_3}\left[ {Al{F_6}} \right]$
$Al{F_3} + 3KF\xrightarrow{{HF}}{K_3}\left[ {Al{F_6}} \right]$ ……. (i)
$Al{F_3}$ is insoluble in anhydrous $HF$ because the ${F^ - }ions$ are not available in intermolecular hydrogen bonded $HF$ but it becomes soluble in the presence of $KF$ due to the formation of soluble complex, ${K_3}\left[ {Al{F_6}} \right]$.
We can understand it from the equation (i).
Therefore, the correct option is (D) ${K_3}\left[ {Al{F_6}} \right]$.
Additional Information:
We can say that –
-Anhydrous $HF$ is a covalent compound and is strongly hydrogen bonded. Therefore, it doesn’t give ${F^ - }ions$ and hence \[Al{F_3}\] doesn’t dissolve in $HF$. $NaF$ is an ionic compound. It contains ${F^ - }ions$ which combine with electron deficient $Al{F_3}$ to form the soluble complex,
$3NaF + Al{F_3} \to N{a_3}\left[ {Al{F_6}} \right]$ (sodium hexafluoroaluminate (iii))
-Due to Boron’s small size and higher electronegativity it has greater tendency to form complexes than Aluminium. Hence, precipitation of \[Al{F_3}\] takes place when $B{F_3}$ is passed through $N{a_3}\left[ {Al{F_6}} \right]$
$N{a_3}\left[ {Al{F_6}} \right] + 3B{F_3} \to 3Na\left[ {B{F_4}} \right] + Al{F_3}$ {sodium tetrafluoroborate (iii)}
Note: ${K_3}\left[ {Al{F_6}} \right]$ (Potassium hexafluoroaluminate) has structure.
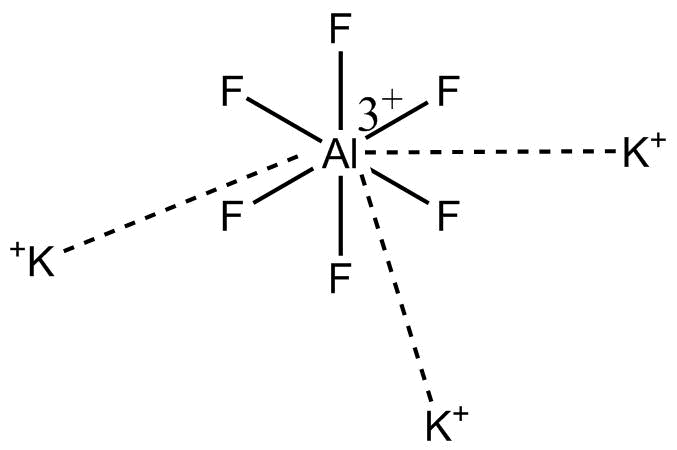
This compound is a compound which can be canonicalized.
Complete step by step answer: Aluminium form fluoride complexes more easily than in the case of silicon. So, $Al{F_3}$ easily dissolves in a mixture of $\left( {HF + KF} \right)$.
$Al{F_3}$ is soluble in $HF$ due to the formation of ${K_3}\left[ {Al{F_6}} \right]$
$Al{F_3} + 3KF\xrightarrow{{HF}}{K_3}\left[ {Al{F_6}} \right]$ ……. (i)
$Al{F_3}$ is insoluble in anhydrous $HF$ because the ${F^ - }ions$ are not available in intermolecular hydrogen bonded $HF$ but it becomes soluble in the presence of $KF$ due to the formation of soluble complex, ${K_3}\left[ {Al{F_6}} \right]$.
We can understand it from the equation (i).
Therefore, the correct option is (D) ${K_3}\left[ {Al{F_6}} \right]$.
Additional Information:
We can say that –
-Anhydrous $HF$ is a covalent compound and is strongly hydrogen bonded. Therefore, it doesn’t give ${F^ - }ions$ and hence \[Al{F_3}\] doesn’t dissolve in $HF$. $NaF$ is an ionic compound. It contains ${F^ - }ions$ which combine with electron deficient $Al{F_3}$ to form the soluble complex,
$3NaF + Al{F_3} \to N{a_3}\left[ {Al{F_6}} \right]$ (sodium hexafluoroaluminate (iii))
-Due to Boron’s small size and higher electronegativity it has greater tendency to form complexes than Aluminium. Hence, precipitation of \[Al{F_3}\] takes place when $B{F_3}$ is passed through $N{a_3}\left[ {Al{F_6}} \right]$
$N{a_3}\left[ {Al{F_6}} \right] + 3B{F_3} \to 3Na\left[ {B{F_4}} \right] + Al{F_3}$ {sodium tetrafluoroborate (iii)}
Note: ${K_3}\left[ {Al{F_6}} \right]$ (Potassium hexafluoroaluminate) has structure.

This compound is a compound which can be canonicalized.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




