
Aldol condensation will not be observed in?
A.Chloral
B.Phenyl acetaldehyde
C.Hexanal
D.Ethanal
Answer
496.8k+ views
Hint: Aldol is the combination of aldehydes and alcohol. The aldol condensation is characterized by the reaction between an aldehyde or ketone with a dilute base. The only important thing is that the aldehyde or ketone should contain an $\alpha -Hydrogen$ atom.
Complete answer:
We know that $\alpha$ hydrogen is the hydrogen present in the carbon atom that is attached to the carbonyl carbon. Now, let us see which compound does not have $\alpha$ hydrogen.
Option A: Chloral
Chloral is also referred to as trichloroacetaldehyde or trichloroethanal. The molecular formula for chloral is $C{{l}_{3}}CCHO$.
Its structural formula is given by:
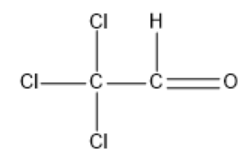
In the above structure, we can observe that the carbon next to the carbonyl carbon does not have any $\alpha $hydrogen. So, this compound cannot undergo an Aldol condensation reaction.
Option B: Phenyl acetaldehyde
Phenyl acetaldehyde has the molecular formula of ${{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO$.
Its structural formula is given by:
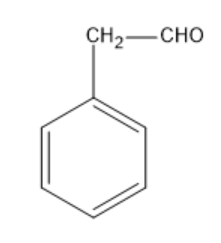
In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Option C: Hexanal
The molecular formula of Hexanal is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}CHO$.
Its structural formula is given by:
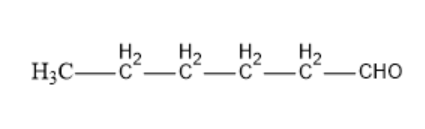
In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Option D: Ethanal
The molecular formula of Hexanal is $C{{H}_{3}}CHO$.
Its structural formula is given by:
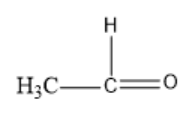
In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Final answer: The correct answer is Option A: Chloral.
Note:
Aldol condensation will not be possible if the $\alpha $hydrogen is absent. Because in Aldol condensation, the $\alpha $ hydrogen attacks the carbonyl carbon to form an $\beta $ Unsaturated carbonyl compound. It is favoured by the loss of water molecules from both reactants.
Complete answer:
We know that $\alpha$ hydrogen is the hydrogen present in the carbon atom that is attached to the carbonyl carbon. Now, let us see which compound does not have $\alpha$ hydrogen.
Option A: Chloral
Chloral is also referred to as trichloroacetaldehyde or trichloroethanal. The molecular formula for chloral is $C{{l}_{3}}CCHO$.
Its structural formula is given by:

In the above structure, we can observe that the carbon next to the carbonyl carbon does not have any $\alpha $hydrogen. So, this compound cannot undergo an Aldol condensation reaction.
Option B: Phenyl acetaldehyde
Phenyl acetaldehyde has the molecular formula of ${{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO$.
Its structural formula is given by:

In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Option C: Hexanal
The molecular formula of Hexanal is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}CHO$.
Its structural formula is given by:

In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Option D: Ethanal
The molecular formula of Hexanal is $C{{H}_{3}}CHO$.
Its structural formula is given by:

In the above structure, we can observe that the carbon next to the carbonyl carbon has $\alpha $hydrogen. So, this compound can undergo an Aldol condensation reaction.
Final answer: The correct answer is Option A: Chloral.
Note:
Aldol condensation will not be possible if the $\alpha $hydrogen is absent. Because in Aldol condensation, the $\alpha $ hydrogen attacks the carbonyl carbon to form an $\beta $ Unsaturated carbonyl compound. It is favoured by the loss of water molecules from both reactants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




