
how many aldehydes are possible with molecular formula ${{C}_{4}}{{H}_{8}}O$ ?
A.$1$
B.$2$
C.$3$
D.$4$
Answer
570.6k+ views
Hint: An aldehyde is defined as a functional group that consists of a carbonyl centre with carbon atoms that are also bonded to the hydrogen and $R$ group. $R$ group can be a hydrogen, alkyl group or any side chain. Molecular formula consists of atoms that have some numeric value as a subscript that tells us about the number of atoms present.
Complete answer:
Molecular formula is defined as the formula that contains chemical symbols of the elements having some numerical subscripts that describes the presence of a number of atoms in a molecule. The limitation of molecular formula is that they are not able to describe the arrangement of the atoms in a molecule. Structural formula is different from molecular formula as it is helpful in describing the number of atoms as well as the arrangement of the atoms in a molecule. In organic compounds, carbon and hydrogen are the first elements in the molecular formula.
Now let us see the structure of the two aldehydes.
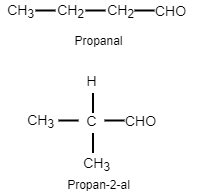
We have seen that two aldehydes are possible with the molecular formula ${{C}_{4}}{{H}_{8}}O$
Hence, the correct option is (B).
Additional information:
A chemical formula is defined as a way of representing the chemical proportions of the atoms that combines together to form a molecule.
Empirical formulas are the simplest type of chemical formula that just only requires numbers and alphabets. Empirical formulas do not contain large numbers. For example, the empirical formula for glucose is $C{{H}_{2}}O$ and molecular formula for glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$
Note: Isomers are defined as the compound having the same molecular formula but different arrangement of atoms. In this question, ${{C}_{4}}{{H}_{8}}O$ are the aldehydes that can be arranged in different forms. This process is known as isomerisation. It is not necessary that the isomers will share the same chemical properties as physical properties.
Complete answer:
Molecular formula is defined as the formula that contains chemical symbols of the elements having some numerical subscripts that describes the presence of a number of atoms in a molecule. The limitation of molecular formula is that they are not able to describe the arrangement of the atoms in a molecule. Structural formula is different from molecular formula as it is helpful in describing the number of atoms as well as the arrangement of the atoms in a molecule. In organic compounds, carbon and hydrogen are the first elements in the molecular formula.
Now let us see the structure of the two aldehydes.

We have seen that two aldehydes are possible with the molecular formula ${{C}_{4}}{{H}_{8}}O$
Hence, the correct option is (B).
Additional information:
A chemical formula is defined as a way of representing the chemical proportions of the atoms that combines together to form a molecule.
Empirical formulas are the simplest type of chemical formula that just only requires numbers and alphabets. Empirical formulas do not contain large numbers. For example, the empirical formula for glucose is $C{{H}_{2}}O$ and molecular formula for glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$
Note: Isomers are defined as the compound having the same molecular formula but different arrangement of atoms. In this question, ${{C}_{4}}{{H}_{8}}O$ are the aldehydes that can be arranged in different forms. This process is known as isomerisation. It is not necessary that the isomers will share the same chemical properties as physical properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




