
Alcohols are compound with the general formula $R-OH$.
a. alcohols are soluble in water. What is the reason?
b. i. Explain a method for manufacture of ethanol?
ii. how will you convert phenol to benzene?
Answer
579k+ views
Hint: Alcohols are a class of compound which contains a hydroxyl group in general, so they are polar in nature, meaning, they have a dipole moment other than zero.
When alcohols undergo reduction, they lose the hydroxyl group, whereas when they undergo oxidation, they are converted to aldehyde and consequently carboxylic acid.
Complete step by step answer:
a.The solubility of alcohol in water is due to the fact that, as we can see from the general formula that alcohol contains a hydroxyl group which is involved in formation of hydrogen bonding with the molecule of water. The interaction can be shown by the given diagram,
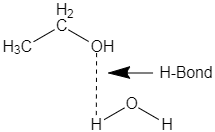
As, we can see that the molecule of alcohol, and water forms a hydrogen bond between them. Moreover, alcohol is also a polar molecule and water is a polar solvent because of the presence of lone pairs in the atom of oxygen.
b.i. If we consider the second part of the question, we can see that it has two subparts, so we will answer each of them individually, so the first subpart asks how we could manufacture ethanol. As we know ethanol contains two carbons in the main chain, so we could take ethene as the reactant, which could react with water in presence of an acid, as a catalyst, in order to form ethanol.
So the reaction could be represented by the following chemical equation,
$C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\xrightarrow{{{H}_{3}}P{{O}_{4}}}C{{H}_{3}}C{{H}_{2}}OH$
As we can see that the ethene molecule reacts with water molecules in presence of phosphoric acid as a catalyst.
ii. in the next part the conversion of phenol, into a benzene ring is being asked. So, we know that a phenol consists of a hydroxyl group attached to a benzene ring. So, we need to remove that hydroxylgroup in order to get benzene.

In the reaction we can see that the phenol is reduced to benzene upon heating with zinc dust.
Note: The alcohols are soluble in water because of the presence of hydrogen bonding between the molecules of alcohol and water.
Ethanol could be manufactured by using ethene, along with water as another reactant in presence of an acid as a catalyst.
Phenol can be converted to benzene, upon reduction with zinc dust.
When alcohols undergo reduction, they lose the hydroxyl group, whereas when they undergo oxidation, they are converted to aldehyde and consequently carboxylic acid.
Complete step by step answer:
a.The solubility of alcohol in water is due to the fact that, as we can see from the general formula that alcohol contains a hydroxyl group which is involved in formation of hydrogen bonding with the molecule of water. The interaction can be shown by the given diagram,

As, we can see that the molecule of alcohol, and water forms a hydrogen bond between them. Moreover, alcohol is also a polar molecule and water is a polar solvent because of the presence of lone pairs in the atom of oxygen.
b.i. If we consider the second part of the question, we can see that it has two subparts, so we will answer each of them individually, so the first subpart asks how we could manufacture ethanol. As we know ethanol contains two carbons in the main chain, so we could take ethene as the reactant, which could react with water in presence of an acid, as a catalyst, in order to form ethanol.
So the reaction could be represented by the following chemical equation,
$C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\xrightarrow{{{H}_{3}}P{{O}_{4}}}C{{H}_{3}}C{{H}_{2}}OH$
As we can see that the ethene molecule reacts with water molecules in presence of phosphoric acid as a catalyst.
ii. in the next part the conversion of phenol, into a benzene ring is being asked. So, we know that a phenol consists of a hydroxyl group attached to a benzene ring. So, we need to remove that hydroxylgroup in order to get benzene.

In the reaction we can see that the phenol is reduced to benzene upon heating with zinc dust.
Note: The alcohols are soluble in water because of the presence of hydrogen bonding between the molecules of alcohol and water.
Ethanol could be manufactured by using ethene, along with water as another reactant in presence of an acid as a catalyst.
Phenol can be converted to benzene, upon reduction with zinc dust.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




