
What is the action of moist silver oxide on ethyl bromide?
Answer
559.2k+ views
Hint: Moist silver oxide acts as a good nucleophile. When it is reacted with ethyl bromide a bimolecular nucleophilic substitution takes place and the product which is formed, is used as a fuel.
Complete step by step answer:
- In order to answer our question, we need to learn about the action of moist silver oxide on alkyl halides. Let us know about the ${{S}_{N}}2$ reaction in detail. Nucleophilic substitution bimolecular or ${{S}_{N}}2$ is a single step bimolecular reaction in which the incoming nucleophile attacks the C-atom of substrate in a direction opposite to the outgoing nucleophile The reaction passes through a transition state in which both the incoming and outgoing nucleophiles are bonded to the same C-atom. In the transition state, the C-atom is $s{{p}^{2}}$ hybridised with a p-orbital whose one lobe overlaps with an orbital of incoming nucleophile and the other lobe overlaps with an orbital of outgoing nucleophile. The three non-reacting atoms or groups attached to the C-atom are nearly coplanar at an angle of ${{120}^{0}}$ The reaction is completed when the outgoing nucleophile leaves with the bond pair of electrons and simultaneously the incoming nucleophile binds to the C-atom. As the reaction progresses, the configuration of the C-atom under attack is inverted. An ${{S}_{N}}2$ reaction is always accompanied by inversion of configuration. The inversion in configuration implies change in configuration from R to S or S to R (provided the incoming nucleophile and outgoing nucleophile have same priority) and not from (+) to (-) or (-) to (+)
Steric hindrance plays a very vital role in an ${{S}_{N}}2$ reaction. As steric hindrance increases the rate of ${{S}_{N}}2$ reaction decreases Thus for the same halogen the reactivity order of alkyl halides towards ${{S}_{N}}2$reaction is as under: methyl halide>primary alkyl halide>secondary alkyl halide>tertiary alkyl halide.
Now, moist silver oxide reacts via ${{S}_{N}}2$ mechanism. We have the reaction as:
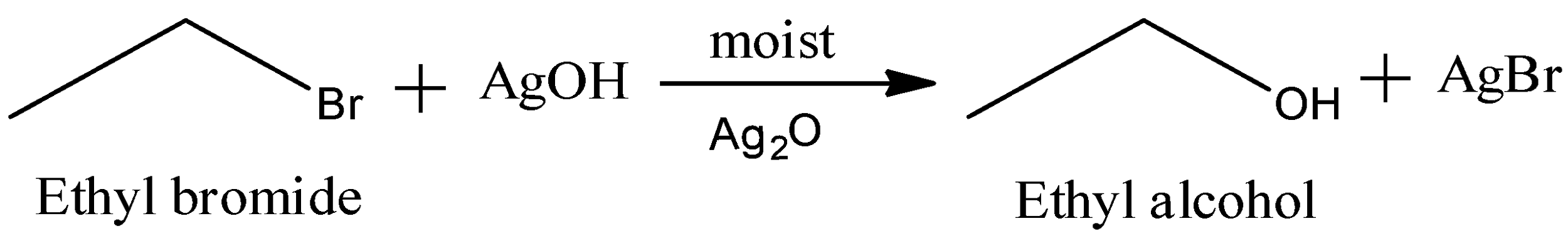
So, we get the major product as ethyl alcohol, and some amount of AgBr is also formed.
Note: For the same alkyl group, the reactivity of alkyl halides increases with the decrease in the $C-X$ bond dissociation energy. Therefore, $R-I>R-Br>R-Cl>R-F$.
Complete step by step answer:
- In order to answer our question, we need to learn about the action of moist silver oxide on alkyl halides. Let us know about the ${{S}_{N}}2$ reaction in detail. Nucleophilic substitution bimolecular or ${{S}_{N}}2$ is a single step bimolecular reaction in which the incoming nucleophile attacks the C-atom of substrate in a direction opposite to the outgoing nucleophile The reaction passes through a transition state in which both the incoming and outgoing nucleophiles are bonded to the same C-atom. In the transition state, the C-atom is $s{{p}^{2}}$ hybridised with a p-orbital whose one lobe overlaps with an orbital of incoming nucleophile and the other lobe overlaps with an orbital of outgoing nucleophile. The three non-reacting atoms or groups attached to the C-atom are nearly coplanar at an angle of ${{120}^{0}}$ The reaction is completed when the outgoing nucleophile leaves with the bond pair of electrons and simultaneously the incoming nucleophile binds to the C-atom. As the reaction progresses, the configuration of the C-atom under attack is inverted. An ${{S}_{N}}2$ reaction is always accompanied by inversion of configuration. The inversion in configuration implies change in configuration from R to S or S to R (provided the incoming nucleophile and outgoing nucleophile have same priority) and not from (+) to (-) or (-) to (+)
Steric hindrance plays a very vital role in an ${{S}_{N}}2$ reaction. As steric hindrance increases the rate of ${{S}_{N}}2$ reaction decreases Thus for the same halogen the reactivity order of alkyl halides towards ${{S}_{N}}2$reaction is as under: methyl halide>primary alkyl halide>secondary alkyl halide>tertiary alkyl halide.
Now, moist silver oxide reacts via ${{S}_{N}}2$ mechanism. We have the reaction as:

So, we get the major product as ethyl alcohol, and some amount of AgBr is also formed.
Note: For the same alkyl group, the reactivity of alkyl halides increases with the decrease in the $C-X$ bond dissociation energy. Therefore, $R-I>R-Br>R-Cl>R-F$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




