
What is the action of bromine in alkaline medium on ${{C}}{{{H}}_{{3}}}{{C}}{{{H}}_{{2}}}{{N}}{{{O}}_{{2}}}$ ?
Answer
570k+ views
Hint: We see the nature of the nitro group here and see the acidity of the adjacent group attached to it to see the most appropriate spot for nucleophiles to attack on the substrate. Then we will see whether excess of reagent affects the product or not.
Complete step by step answer:
Since we know the nitro group is an electron withdrawing group. Because of this electron withdrawing nature it shifts the electron of adjacent carbon toward itself making that adjacent carbon an electron-deficient carbon. The hydrogens present on the electron deficient carbon become very acidic and can be easily removed as protons by attack of nucleophiles. Here the nucleophile is bromide ion.
The reaction is as follows
In STEP ${{1}}$ the acidic proton or hydrogen is released from the ${{\alpha - carbon}}$ and the nucleophile is attached to it.
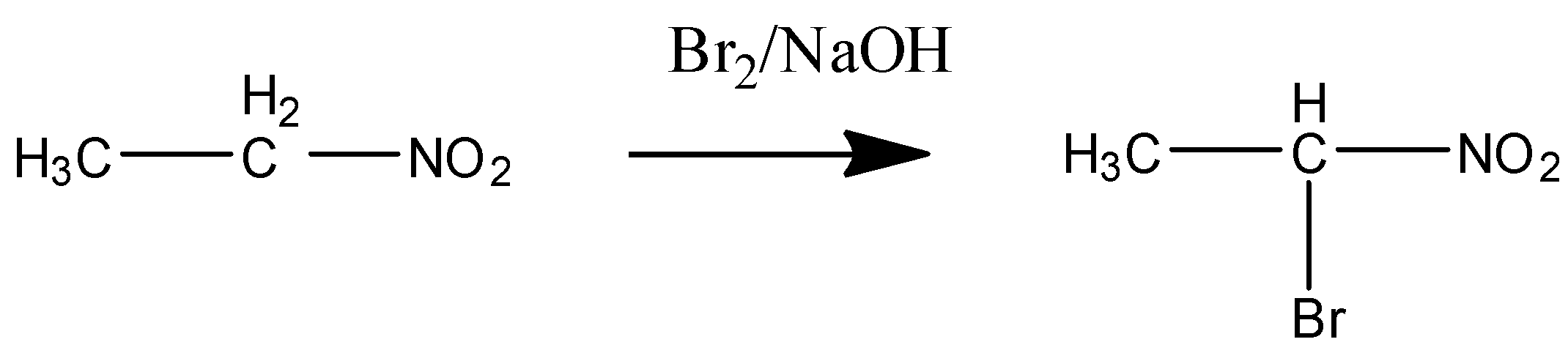
Bromine in NaOH is the alkaline medium as a condition given.
STEP ${{2}}$ when excess or more amount of reagent is present in the solution then the other ${{\alpha - hydrogen}}$of the carbon is also removed and the di-bromo product is formed. The following step is shown below:
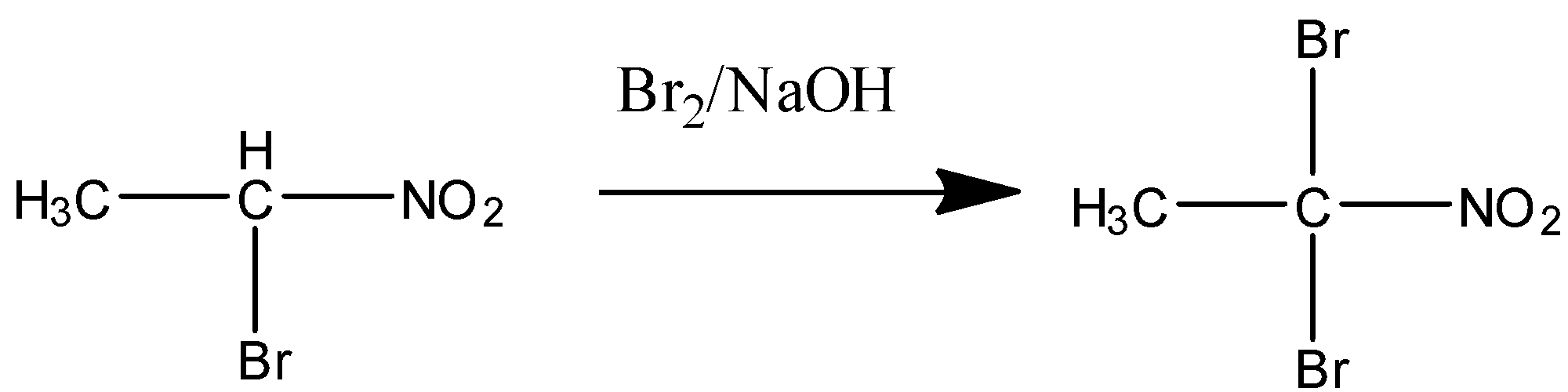
${{ \alpha - bromonitroethane}} \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\;
\;\;\;\; \;\;\;\; \;\;\;\; {\text{1,1 - dibromonitroethane}}$
This is the product of nitroethane in alkaline bromine. The presence of base here facilitates the removal of acidic protons from the adjacent carbon. The primary nitroalkanes and the secondary nitroalkanes can be very easily converted to ${{\alpha - brominated alkane}}$ or any halogen in place of bromine. The primary nitroalkanes can be converted to mono or di-halogenated nitroalkanes but the secondary nitroalkanes can only be converted to mono-halogenated nitroalkane even if there is excess reagent present.
Additional information:.
The reaction also produces side products. They are sodium bromide if NaOH is taken, potassium bromide if KOH was taken as a base and water is also released.
Note: Bromine is a brown colored solution, when equivalent quantities of nitroalkane is mixed with the alkali the bromine is added in drops. This makes the color of solution disappear and when it is cooled and more addition of bromine makes the solution yellow in color.
Complete step by step answer:
Since we know the nitro group is an electron withdrawing group. Because of this electron withdrawing nature it shifts the electron of adjacent carbon toward itself making that adjacent carbon an electron-deficient carbon. The hydrogens present on the electron deficient carbon become very acidic and can be easily removed as protons by attack of nucleophiles. Here the nucleophile is bromide ion.
The reaction is as follows
In STEP ${{1}}$ the acidic proton or hydrogen is released from the ${{\alpha - carbon}}$ and the nucleophile is attached to it.
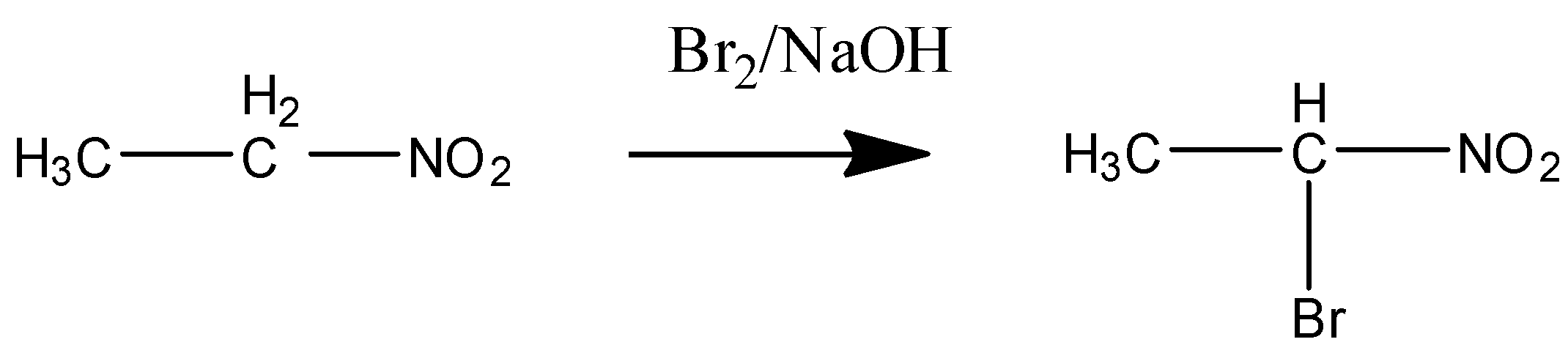
Bromine in NaOH is the alkaline medium as a condition given.
STEP ${{2}}$ when excess or more amount of reagent is present in the solution then the other ${{\alpha - hydrogen}}$of the carbon is also removed and the di-bromo product is formed. The following step is shown below:

${{ \alpha - bromonitroethane}} \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\; \;\;\;\;
\;\;\;\; \;\;\;\; \;\;\;\; {\text{1,1 - dibromonitroethane}}$
This is the product of nitroethane in alkaline bromine. The presence of base here facilitates the removal of acidic protons from the adjacent carbon. The primary nitroalkanes and the secondary nitroalkanes can be very easily converted to ${{\alpha - brominated alkane}}$ or any halogen in place of bromine. The primary nitroalkanes can be converted to mono or di-halogenated nitroalkanes but the secondary nitroalkanes can only be converted to mono-halogenated nitroalkane even if there is excess reagent present.
Additional information:.
The reaction also produces side products. They are sodium bromide if NaOH is taken, potassium bromide if KOH was taken as a base and water is also released.
Note: Bromine is a brown colored solution, when equivalent quantities of nitroalkane is mixed with the alkali the bromine is added in drops. This makes the color of solution disappear and when it is cooled and more addition of bromine makes the solution yellow in color.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




