
How many acids can we use to make iso-butane by decarboxylation?
A. $ 4 $
B. $ 3 $
C. $ 2 $
D. $ 5 $
Answer
515.4k+ views
Hint: An organic reaction in which removal of substituent groups takes place, is known as elimination reaction. Decarboxylation reaction is the type of elimination reaction in which a carboxylic acid is reduced to respective alkane with the removal of carbon dioxide.
Complete answer:
The structure of given compound i.e., isobutane is as follows:
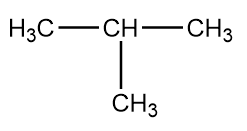
The formation of isobutane by the decarboxylation reactions can take place in following ways:
Preparation Reaction-1:
When 2-methylbutanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
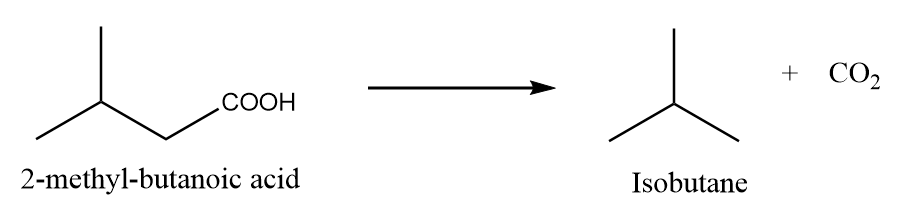
Preparation Reaction-2:
When 2,2-dimethylpropanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
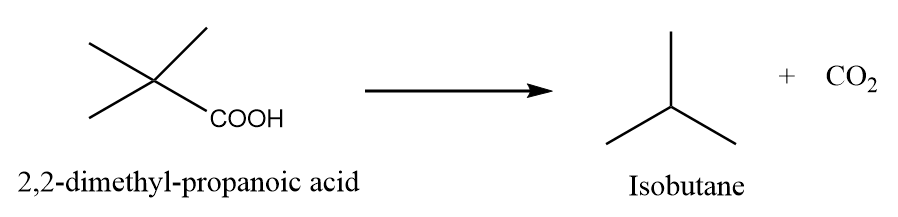
Preparation Reaction-3:
When 3-methylpentanedioic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
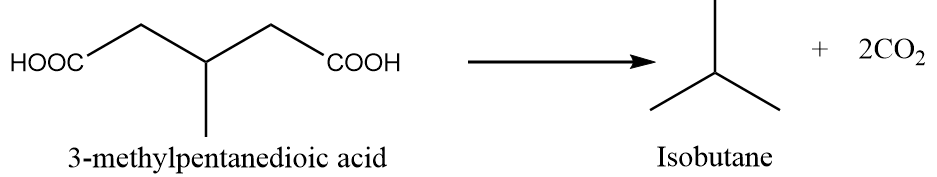
Preparation Reaction-4:
When 2,2-dimethylbutanedioc acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
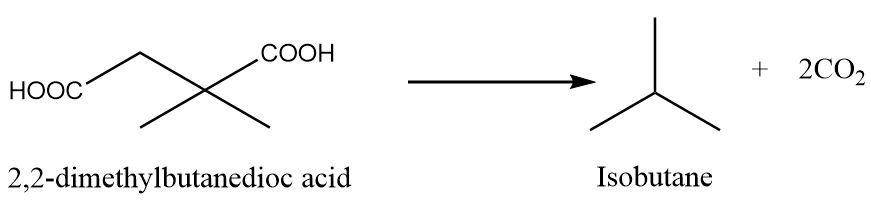
Preparation Reaction-5:
When 2-methylpropane-1,2,3-tricarboxylic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
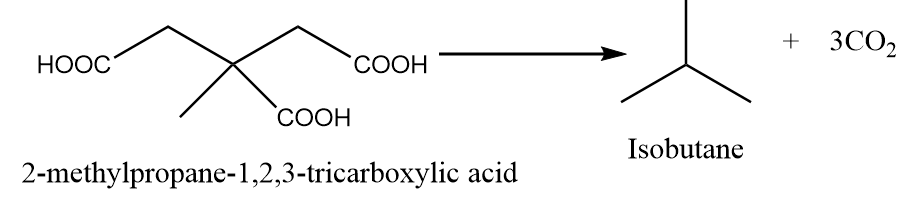
Hence, five acids can be used to form isobutane via decarboxylation reaction.
Thus, option (D) is the correct answer.
Note:
It is important to note that in actuality, there are a total seven acids through which isobutane can be formed via decarboxylation reactions which are two monobasic acids, three dibasic acids and two tribasic acids. It is considered as the most important reaction for the formation of alkanes.
Complete answer:
The structure of given compound i.e., isobutane is as follows:

The formation of isobutane by the decarboxylation reactions can take place in following ways:
Preparation Reaction-1:
When 2-methylbutanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:

Preparation Reaction-2:
When 2,2-dimethylpropanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:

Preparation Reaction-3:
When 3-methylpentanedioic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:

Preparation Reaction-4:
When 2,2-dimethylbutanedioc acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
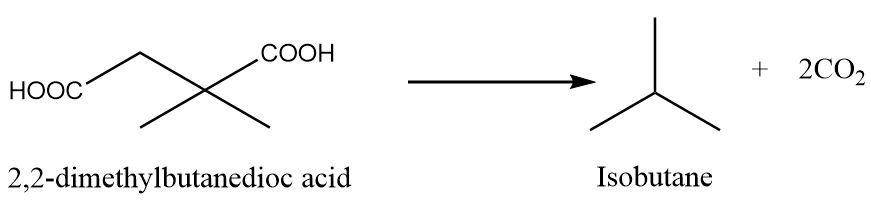
Preparation Reaction-5:
When 2-methylpropane-1,2,3-tricarboxylic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:

Hence, five acids can be used to form isobutane via decarboxylation reaction.
Thus, option (D) is the correct answer.
Note:
It is important to note that in actuality, there are a total seven acids through which isobutane can be formed via decarboxylation reactions which are two monobasic acids, three dibasic acids and two tribasic acids. It is considered as the most important reaction for the formation of alkanes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




