
Acid present in tamarind is:
(A) Lactic acid
(B) Citric acid
(C) Tartaric acid
(D) Acetic acid
Answer
540.3k+ views
Hint :So, we know that tamarind is a hardwood tree which produces bean-like pods filled with seeds surrounded by fibrous pulp. The pulp of young fruit is green and sour. The sourness comes from the acid present in it. Also, tamarind is edible so it means that the acid present in it will be a weak acid.
Complete Step By Step Answer:
The acid present in tamarind is the tartaric acid. Tartaric acid is also known as dihydroxybutane-dioic acid. It is mainly found in fruits like grapes, bananas, tamarind etc. As its IUPAC name suggests, it will therefore be made up of four carbons, two alcoholic groups and two carboxylic acid groups attached to the carbon chain.
So, the molecular formula of Tartaric acid is: $ {C_4}{H_6}{O_6} $
The chemical formula will be as: $ {(CH(OH)COOH)_2} $
Therefore, the structural formula of Tartaric acid will be:
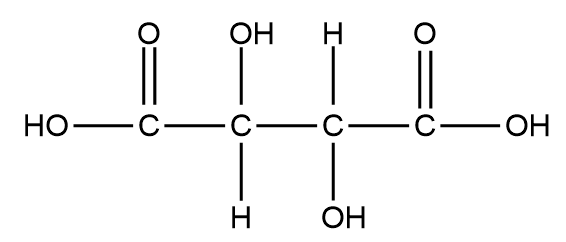
Some uses of tartaric acid:
It is readily soluble in water and is used in food industries due to its antioxidant property.
It is used for polishing metals, photographic printing, wool dyeing etc.
Cream of tartar is incorporated into baking powder, hard candies etc.
It is used as an acidulant in grape and lime-flavored beverages, gelatin desserts, jams, jellies etc.
Hence the correct answer is Option C. i.e., Tartaric acid.
Note :
Tartaric acid is a weak acid because it does not completely dissociate in the solution and also it has a $ pH $ of $ 3.5 $ . It dissociates into bitartrate and tartrate ions. Also, Tartaric acid is a chiral molecule and will thus form stereoisomers. Thus, tartaric acid forms three stereoisomers namely D-Tartaric acid, L-Tartaric acid and meso- Tartaric acid.
Complete Step By Step Answer:
The acid present in tamarind is the tartaric acid. Tartaric acid is also known as dihydroxybutane-dioic acid. It is mainly found in fruits like grapes, bananas, tamarind etc. As its IUPAC name suggests, it will therefore be made up of four carbons, two alcoholic groups and two carboxylic acid groups attached to the carbon chain.
So, the molecular formula of Tartaric acid is: $ {C_4}{H_6}{O_6} $
The chemical formula will be as: $ {(CH(OH)COOH)_2} $
Therefore, the structural formula of Tartaric acid will be:

Some uses of tartaric acid:
It is readily soluble in water and is used in food industries due to its antioxidant property.
It is used for polishing metals, photographic printing, wool dyeing etc.
Cream of tartar is incorporated into baking powder, hard candies etc.
It is used as an acidulant in grape and lime-flavored beverages, gelatin desserts, jams, jellies etc.
Hence the correct answer is Option C. i.e., Tartaric acid.
Note :
Tartaric acid is a weak acid because it does not completely dissociate in the solution and also it has a $ pH $ of $ 3.5 $ . It dissociates into bitartrate and tartrate ions. Also, Tartaric acid is a chiral molecule and will thus form stereoisomers. Thus, tartaric acid forms three stereoisomers namely D-Tartaric acid, L-Tartaric acid and meso- Tartaric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




