
Acetic acid exists as dimer in benzene due to:
A.Condensation reaction
B.Hydrogen bonding
C.Presence of carboxyl group
D.Presence of hydrogen atom at ${{\alpha }}$ -carbon
Answer
590.1k+ views
Hint:Those organic compounds which contain carboxyl groups as the functional group are called carboxylic acids. The carboxyl group itself is made up of a carbonyl group and a hydroxyl group. The ‘carb’ part of the name comes from carbonyl and the ‘oxyl’ part of the name comes from hydroxyl.
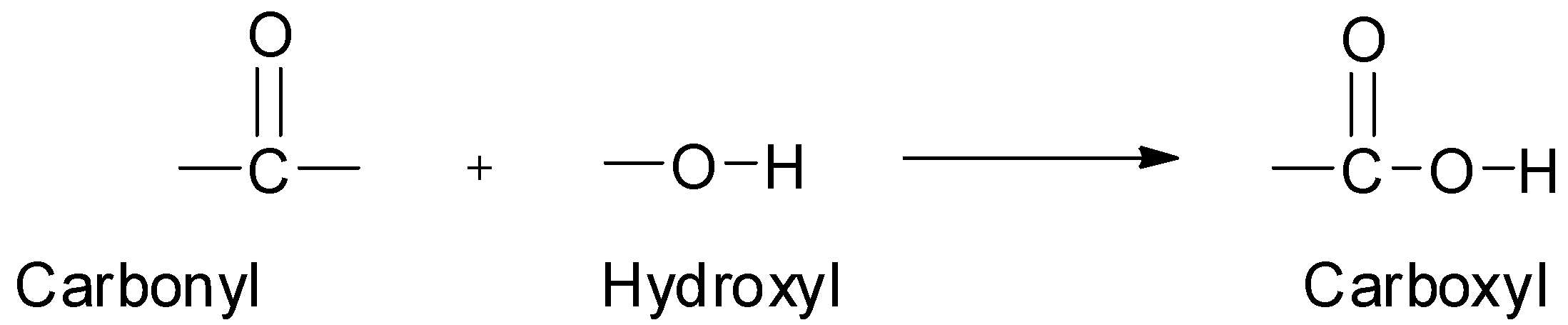
Complete step by step answer:
It can be prepared by the oxidation of ethyl alcohol or ethanol with oxidizing agents like potassium permanganate in an alkaline medium. Under these conditions, the potassium salt is first formed which on treatment with dilute sulphuric acid gives the acetic acid.
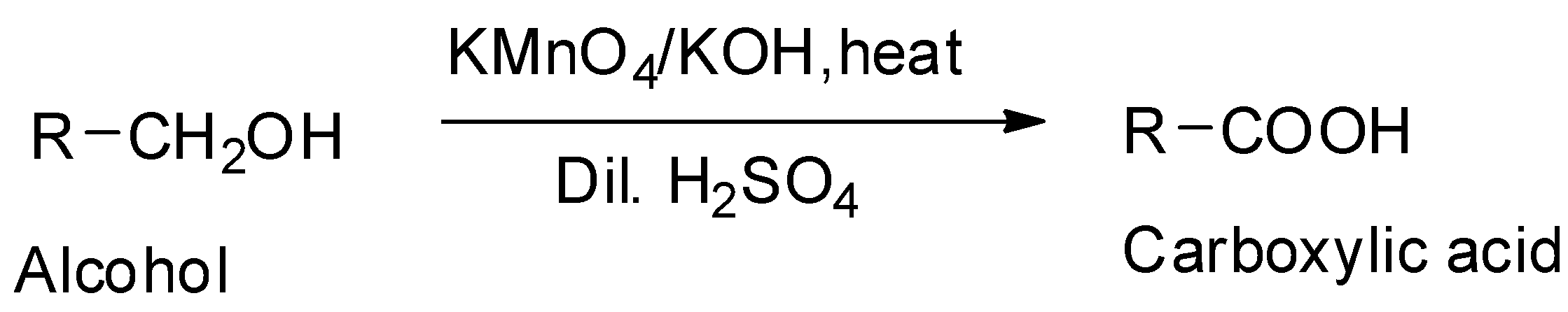
The oxygen – hydrogen bond in carboxylic acids is more strongly polarized than that in alcohols due to the adjacent electron withdrawing carbonyl group. So, the carboxylic acids can form stronger hydrogen bonds.
The negatively polarized oxygen atom of the carbonyl group can also form hydrogen bonds with the positively polarized hydrogen atom of the oxygen – hydrogen bond of a second carboxylic acid molecule. Thus, in case of acetic acid also, these kinds of hydrogen bonds are present.
These hydrogen bonds are so strong that they cannot be broken even in the gas phase. In the gas or vapor phase and also in aprotic solvents like benzene, most of the carboxylic acids exist as cyclic dimers in which two molecules of the acid are held together by two strong hydrogen bonds. Thus, acetic acid also exists as a cyclic dimer in which two of its molecules are held by two strong hydrogen bonds.
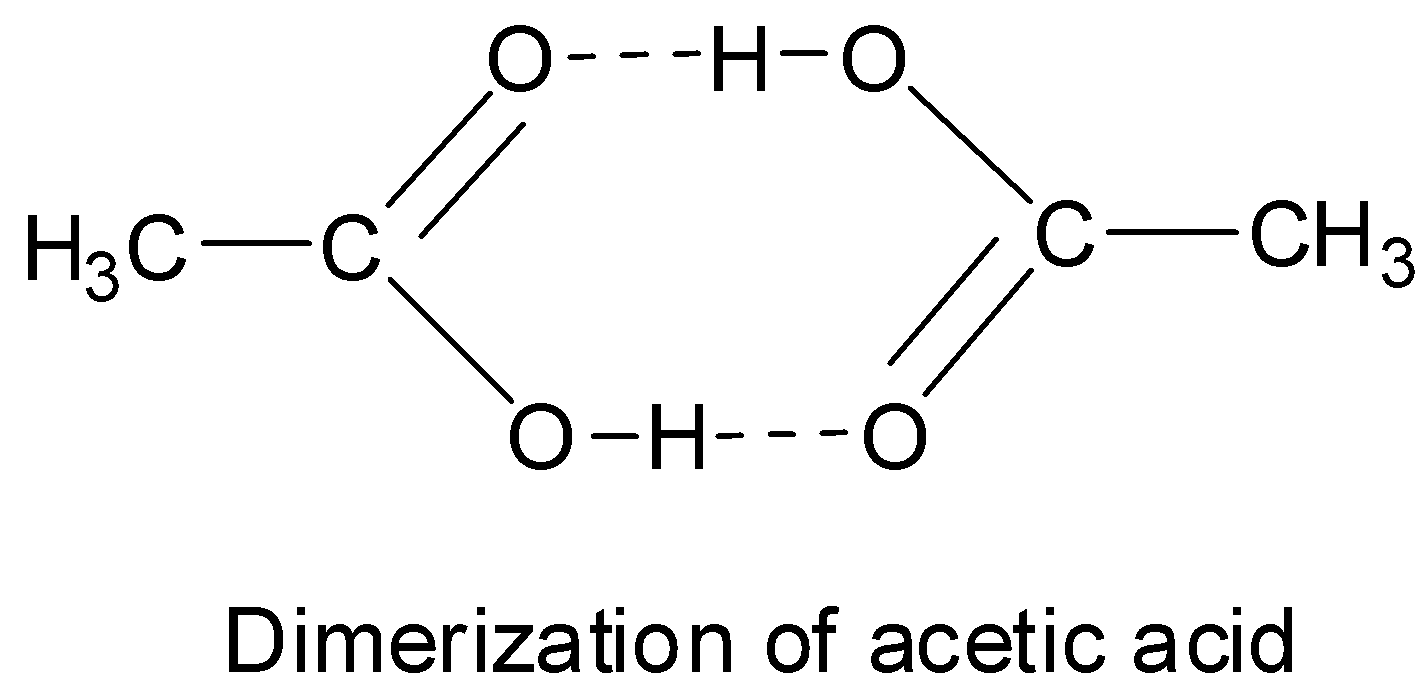
The existence of this cyclic dimer is proved by the fact that the molecular mass of acetic acid in solvents like benzene as determined by measurement of colligative properties is found to be 120 instead of 60. The electron diffraction studies have also confirmed the presence of an eight-membered ring.
So, acetic acid exists as dimer in benzene due to hydrogen bonding and so only option B is correct and the other options are wrong.
Hence option B is correct.
Note:
There are many important uses of acetic acid. Some of these are:
1.It is used as a chemical reagent in the production of many chemical compounds.
2.The most important use of acetic acid is in the production of vinyl acetate monomers which are then polymerized to polyvinyl acetate polymer.
3.The esters of acetic acid are commonly used as solvents for inks, coatings and paints. 4.These esters include ethyl acetate, propyl acetate, butyl acetate and isobutyl acetate.
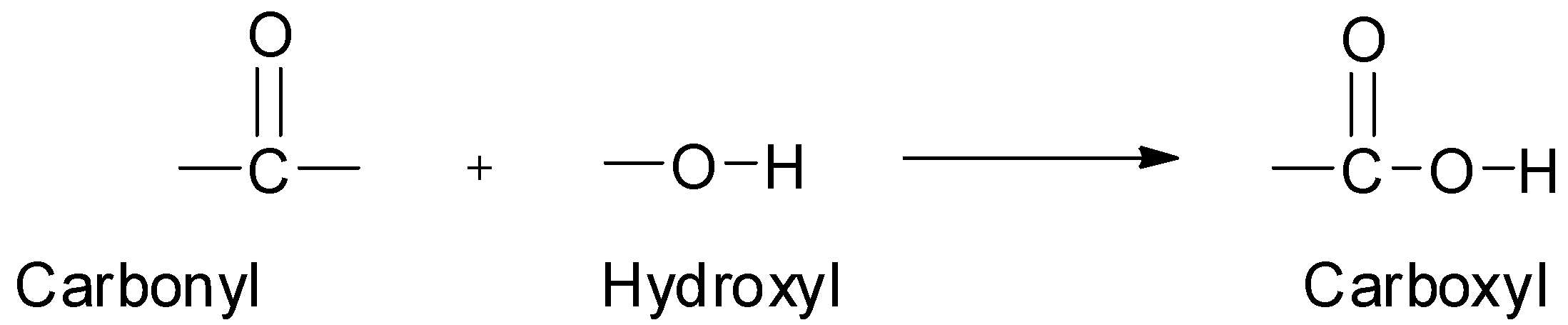
Complete step by step answer:
It can be prepared by the oxidation of ethyl alcohol or ethanol with oxidizing agents like potassium permanganate in an alkaline medium. Under these conditions, the potassium salt is first formed which on treatment with dilute sulphuric acid gives the acetic acid.
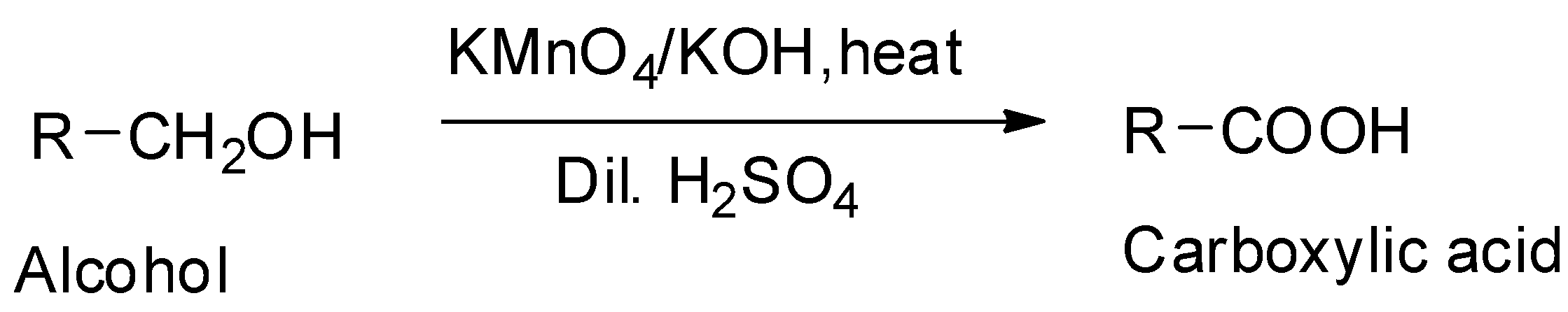
The oxygen – hydrogen bond in carboxylic acids is more strongly polarized than that in alcohols due to the adjacent electron withdrawing carbonyl group. So, the carboxylic acids can form stronger hydrogen bonds.
The negatively polarized oxygen atom of the carbonyl group can also form hydrogen bonds with the positively polarized hydrogen atom of the oxygen – hydrogen bond of a second carboxylic acid molecule. Thus, in case of acetic acid also, these kinds of hydrogen bonds are present.
These hydrogen bonds are so strong that they cannot be broken even in the gas phase. In the gas or vapor phase and also in aprotic solvents like benzene, most of the carboxylic acids exist as cyclic dimers in which two molecules of the acid are held together by two strong hydrogen bonds. Thus, acetic acid also exists as a cyclic dimer in which two of its molecules are held by two strong hydrogen bonds.

The existence of this cyclic dimer is proved by the fact that the molecular mass of acetic acid in solvents like benzene as determined by measurement of colligative properties is found to be 120 instead of 60. The electron diffraction studies have also confirmed the presence of an eight-membered ring.
So, acetic acid exists as dimer in benzene due to hydrogen bonding and so only option B is correct and the other options are wrong.
Hence option B is correct.
Note:
There are many important uses of acetic acid. Some of these are:
1.It is used as a chemical reagent in the production of many chemical compounds.
2.The most important use of acetic acid is in the production of vinyl acetate monomers which are then polymerized to polyvinyl acetate polymer.
3.The esters of acetic acid are commonly used as solvents for inks, coatings and paints. 4.These esters include ethyl acetate, propyl acetate, butyl acetate and isobutyl acetate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




