
Acetaldehyde forms white crystalline precipitate on mixing with a ________solution of __________.
(A) Acidic Zn, Hg
(B) Alcoholic $N{{a}_ {2}} S{{O}_ {3}} $
(C) Saturated, aqueous $NaHS{{O}_ {3}} $
(D) Aqueous NaCl
Answer
576.6k+ views
Hint:. Acetaldehyde is an organic chemical compound, sometimes abbreviated by chemists as $MeCHO$(Me= methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
Complete step by step answer:
We have been provided with acetaldehyde,
Acetaldehyde is a very reactive compound and toxic to the body, your body has an efficient mechanism for handling that. It changes it into a very stable compound, which is acetate.
If we would add sodium bisulfite to acetaldehyde,
Sodium bisulphite is a chemical mixture with the approximate chemical formula $NaHS{{O}_ {3}} $. Sodium bisulphite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It is a white solid with an odour of sulphur dioxide.
When acetone is added to the saturated sodium bisulfite solution, both of them undergo an addition reaction to form a white addition product that is insoluble in a saturated sodium bisulfite solution.
It changes into white crystalline precipitate,
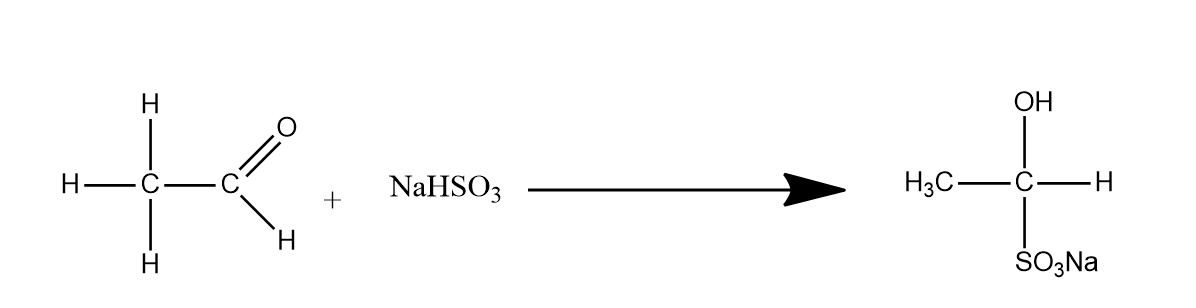
So, we can say that: Acetaldehyde forms white crystalline precipitate on mixing with a saturated solution of aqueous $NaHS{{O}_ {3}} $.
So, the correct answer is “Option C”.
Note: Sulphites are a preservative many people are sensitive to that can severely aggravate asthma. Their use on fresh fruits and vegetables is banned in the United States, but sulphites are present in other foods. (Avoid products listing sulphur dioxide, potassium bisulphite, sodium bisulfite or sodium sulphite on the label.)
Complete step by step answer:
We have been provided with acetaldehyde,
Acetaldehyde is a very reactive compound and toxic to the body, your body has an efficient mechanism for handling that. It changes it into a very stable compound, which is acetate.
If we would add sodium bisulfite to acetaldehyde,
Sodium bisulphite is a chemical mixture with the approximate chemical formula $NaHS{{O}_ {3}} $. Sodium bisulphite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It is a white solid with an odour of sulphur dioxide.
When acetone is added to the saturated sodium bisulfite solution, both of them undergo an addition reaction to form a white addition product that is insoluble in a saturated sodium bisulfite solution.
It changes into white crystalline precipitate,
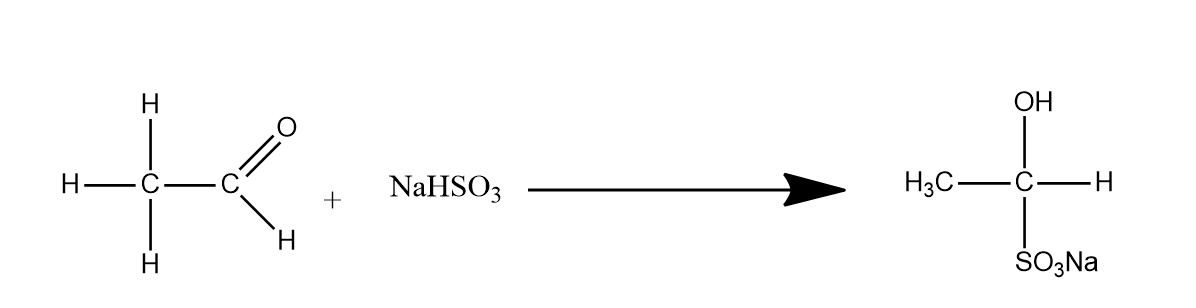
So, we can say that: Acetaldehyde forms white crystalline precipitate on mixing with a saturated solution of aqueous $NaHS{{O}_ {3}} $.
So, the correct answer is “Option C”.
Note: Sulphites are a preservative many people are sensitive to that can severely aggravate asthma. Their use on fresh fruits and vegetables is banned in the United States, but sulphites are present in other foods. (Avoid products listing sulphur dioxide, potassium bisulphite, sodium bisulfite or sodium sulphite on the label.)
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




