
Account on the statement, ethylamine is soluble in water, whereas aniline is insoluble.
Answer
522.6k+ views
Hint: Ethylamine and aniline both compounds are members of the same group, i.e., amines, since both the compounds are organic, we can classify them as soluble or not on the basis of the presence of hydrocarbon part in the compound.
Complete answer:
There are many properties of the substance and one of the properties is the solubility of the given compound in the water, as water is a universal solvent. If the compound is hydrophilic then it will be soluble in water and if the compound is hydrophobic then it will not be soluble in water.
So, in the question, it is given that ethylamine is soluble in water but aniline is insoluble. Ethylamine and aniline both compounds that are members of the same group, i.e., amines, since both the compounds are organic, we can classify them as soluble or not on the basis of the presence of hydrocarbon part in the compound.
The formula of ethylamine is $C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$ and the structure of aniline is:
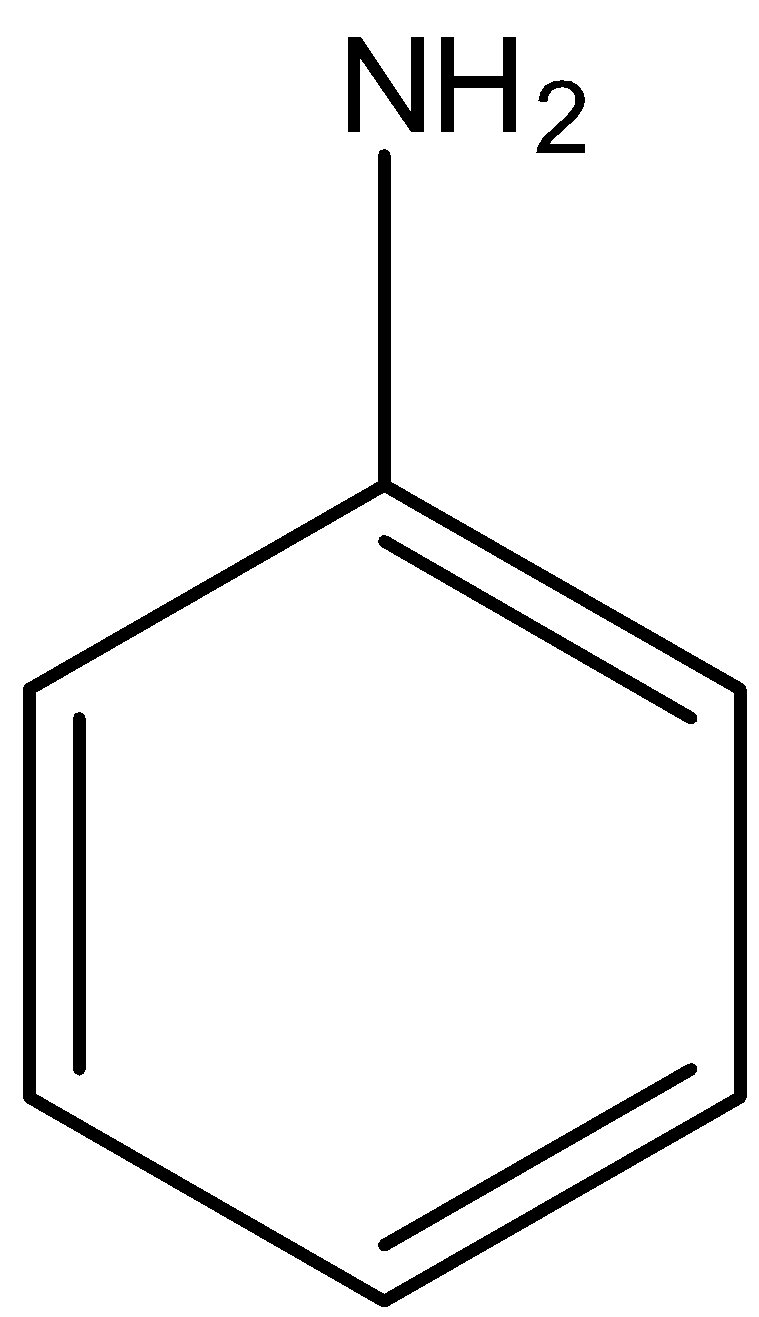
Since both have nitrogen atoms which is an electronegative atom that has the ability to form hydrogen bonding in water. The hydrocarbon part in the ethylamine is ethyl group, i.e., $C{{H}_{3}}C{{H}_{2}}-$ and the hydrocarbon part in aniline is phenyl, i.e., ${{C}_{6}}{{H}_{5}}-$. As the hydrocarbon part becomes bigger, the solubility decreases. Hence, aniline is insoluble in water whereas ethylamine is soluble.
Note:
The hydrocarbon part in the organic compound has only the ability to form weak van der Waal forces which decreases the hydrogen bonding in the water. Hydrogen bonding is a stronger bond than the van der Waal forces.
Complete answer:
There are many properties of the substance and one of the properties is the solubility of the given compound in the water, as water is a universal solvent. If the compound is hydrophilic then it will be soluble in water and if the compound is hydrophobic then it will not be soluble in water.
So, in the question, it is given that ethylamine is soluble in water but aniline is insoluble. Ethylamine and aniline both compounds that are members of the same group, i.e., amines, since both the compounds are organic, we can classify them as soluble or not on the basis of the presence of hydrocarbon part in the compound.
The formula of ethylamine is $C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$ and the structure of aniline is:

Since both have nitrogen atoms which is an electronegative atom that has the ability to form hydrogen bonding in water. The hydrocarbon part in the ethylamine is ethyl group, i.e., $C{{H}_{3}}C{{H}_{2}}-$ and the hydrocarbon part in aniline is phenyl, i.e., ${{C}_{6}}{{H}_{5}}-$. As the hydrocarbon part becomes bigger, the solubility decreases. Hence, aniline is insoluble in water whereas ethylamine is soluble.
Note:
The hydrocarbon part in the organic compound has only the ability to form weak van der Waal forces which decreases the hydrogen bonding in the water. Hydrogen bonding is a stronger bond than the van der Waal forces.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




