
According to the molecular orbital theory, ${{\rm{C}}_{\rm{2}}}$ molecule has:
A. $1\sigma $ and $1\pi $ bond
B. Only $2\sigma $ bonds
C. Only $2\pi $ bonds
D. $1\sigma $ and $2\pi $ bond
Answer
566.4k+ views
Hint: We can deduce the bonding in a molecule by looking at the molecular orbital diagram for the same as to whether sigma or pi- molecular orbitals are being used for bonding.
Step by step answer: We have various theories for bonding and here we will take up the molecular orbital theory. We can use the molecular orbital theory for drawing molecular orbital diagrams for a molecule which is quite useful in deducing various properties such as bond order or explaining magnetic behavior of the molecule. Let’s have a look at the molecular orbital diagram of \[{{\rm{C}}_{\rm{2}}}\] molecule that is given below:
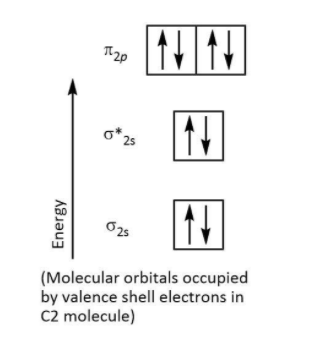
We know that one carbon atom has $6$ valence electrons which mean a \[{{\rm{C}}_{\rm{2}}}\] molecule has $12$ electrons in the molecular orbitals. We can write the electronic configuration for \[{{\rm{C}}_{\rm{2}}}\] molecule with $12$ electrons as follows:
${\left( {{\sigma _{1s}}} \right)^2}{\left( {\sigma _{1s}^*} \right)^2}{\left( {{\sigma _{2s}}} \right)^2}{\left( {\sigma _{2s}^*} \right)^2}\left( {\pi _{2{p_x}}^2 = \pi _{2{p_y}}^2} \right)$
Now, we can determine the bond order for \[{{\rm{C}}_{\rm{2}}}\] molecule by using the following formula:
$B.O. = \dfrac{{{{\rm{N}}_{{\rm{electrons in bonding}}\;{\rm{MO}}}} - {{\rm{N}}_{{\rm{electrons in anti - bonding}}\;{\rm{MO}}}}}}{2}$
As we can see from the electronic configuration for \[{{\rm{C}}_{\rm{2}}}\] molecule, we have $8$ electrons in bonding molecular orbitals and $4$ electrons in antibonding molecular orbitals. Let’s calculate the bond order by substituting these values in the above formula as follows:
$
B.O. = \dfrac{{{\rm{8}} - {\rm{4}}}}{2}\\
\Rightarrow B.O = 2
$
So, we can say that the two carbon atoms are connected by a double bond.
Now let’s consider this double bond. We can see there are $4$ electrons present in $2\pi $ molecular orbitals which means that the double bond is made of $2\pi $ bonds.
Hence, the correct option is C.
Note: Usually, we have known that a double bond is consisted of $1\sigma $ and $1\pi $ bond but this in true case of \[{{\rm{C}}_{\rm{2}}}\] molecule. So, we need to consider the occupancy of the molecular orbitals as well.
Step by step answer: We have various theories for bonding and here we will take up the molecular orbital theory. We can use the molecular orbital theory for drawing molecular orbital diagrams for a molecule which is quite useful in deducing various properties such as bond order or explaining magnetic behavior of the molecule. Let’s have a look at the molecular orbital diagram of \[{{\rm{C}}_{\rm{2}}}\] molecule that is given below:

We know that one carbon atom has $6$ valence electrons which mean a \[{{\rm{C}}_{\rm{2}}}\] molecule has $12$ electrons in the molecular orbitals. We can write the electronic configuration for \[{{\rm{C}}_{\rm{2}}}\] molecule with $12$ electrons as follows:
${\left( {{\sigma _{1s}}} \right)^2}{\left( {\sigma _{1s}^*} \right)^2}{\left( {{\sigma _{2s}}} \right)^2}{\left( {\sigma _{2s}^*} \right)^2}\left( {\pi _{2{p_x}}^2 = \pi _{2{p_y}}^2} \right)$
Now, we can determine the bond order for \[{{\rm{C}}_{\rm{2}}}\] molecule by using the following formula:
$B.O. = \dfrac{{{{\rm{N}}_{{\rm{electrons in bonding}}\;{\rm{MO}}}} - {{\rm{N}}_{{\rm{electrons in anti - bonding}}\;{\rm{MO}}}}}}{2}$
As we can see from the electronic configuration for \[{{\rm{C}}_{\rm{2}}}\] molecule, we have $8$ electrons in bonding molecular orbitals and $4$ electrons in antibonding molecular orbitals. Let’s calculate the bond order by substituting these values in the above formula as follows:
$
B.O. = \dfrac{{{\rm{8}} - {\rm{4}}}}{2}\\
\Rightarrow B.O = 2
$
So, we can say that the two carbon atoms are connected by a double bond.
Now let’s consider this double bond. We can see there are $4$ electrons present in $2\pi $ molecular orbitals which means that the double bond is made of $2\pi $ bonds.
Hence, the correct option is C.
Note: Usually, we have known that a double bond is consisted of $1\sigma $ and $1\pi $ bond but this in true case of \[{{\rm{C}}_{\rm{2}}}\] molecule. So, we need to consider the occupancy of the molecular orbitals as well.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




