
According to Lothar Meyer’s curve, which of the following statements is/are correct?
This question has multiple correct options
(A)- The elements having the same properties will occupy the same position in the curve.
(B)- Alkaline Earth metals are at the peaks of the curve.
(C)- Halogens are the ascending part of the curve.
(D)- The atomic volumes of elements in a period initially decrease and then increase.
Answer
566.7k+ views
Hint: Lothar Meyer arranged the elements according to the similarity in properties. Meyer also took into account the particular positions on the curve assigning to a particular group of elements in consideration with their similarities.
Complete Solution :
-Lothar Meyer was the first person to observe periodic trends in the properties of elements.
-He plotted a graph between the atomic mass and physical properties of the elements (viz. Atomic volume, boiling point, melting point etc..).
-Unlike Newlands (Newland’s law of octaves) he observed a change in length, the repeating pattern of properties.
-Given below is the simple representation of Lothar Meyer’s plot (curve).
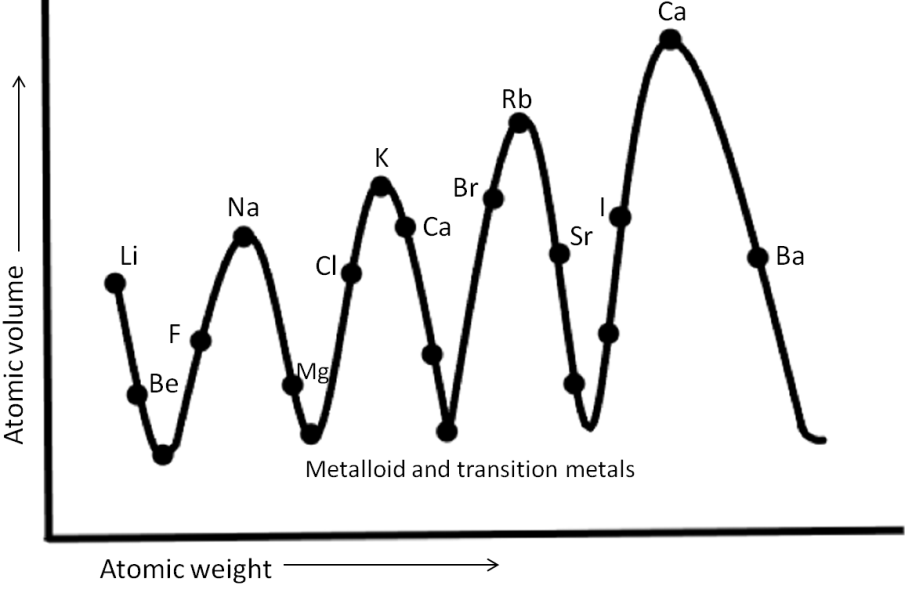
-Lothar Meyer proposed that by arranging the elements in order of increasing atomic weights; similarities appear in physical and chemical properties.
-From this curve, it was observed that the elements having similar properties, occupied the same corresponding position on the curve.
-It is evident from the curve that halogens, since they share similar properties, are placed at a similar position, ascending part of the curve.
-Alkali metals, as they are sharing similar properties are located at the peak of the curves. Whereas alkaline earth metals are placed at the descending part of the curves.
-Looking at the graph, we can observe that along a particular period, the atomic volume of elements initially decreases and then increases.
-From the above observations, it is clear that Option (B) Alkaline Earth metals are at the peaks of the curve, is an incorrect option since we know that alkaline earth metals are placed at the descending part of the curve, whereas alkaline earth metals are placed at the peaks of the curve.
Hence, Option (A) The elements having the same properties will occupy the same position in the curve, Option (C) Halogens are the ascending part of the curve, And Option (D) The atomic volumes of elements in a period initially decrease and then increase are the correct answers.
So, the correct answer is “Option D”.
Note: Noble gases like Ne, Ar, Kr, which are another group of elements with similar properties, are placed just before alkali metals in every curve and are located in the ascending part of the curve along with halogens.
Complete Solution :
-Lothar Meyer was the first person to observe periodic trends in the properties of elements.
-He plotted a graph between the atomic mass and physical properties of the elements (viz. Atomic volume, boiling point, melting point etc..).
-Unlike Newlands (Newland’s law of octaves) he observed a change in length, the repeating pattern of properties.
-Given below is the simple representation of Lothar Meyer’s plot (curve).

-Lothar Meyer proposed that by arranging the elements in order of increasing atomic weights; similarities appear in physical and chemical properties.
-From this curve, it was observed that the elements having similar properties, occupied the same corresponding position on the curve.
-It is evident from the curve that halogens, since they share similar properties, are placed at a similar position, ascending part of the curve.
-Alkali metals, as they are sharing similar properties are located at the peak of the curves. Whereas alkaline earth metals are placed at the descending part of the curves.
-Looking at the graph, we can observe that along a particular period, the atomic volume of elements initially decreases and then increases.
-From the above observations, it is clear that Option (B) Alkaline Earth metals are at the peaks of the curve, is an incorrect option since we know that alkaline earth metals are placed at the descending part of the curve, whereas alkaline earth metals are placed at the peaks of the curve.
Hence, Option (A) The elements having the same properties will occupy the same position in the curve, Option (C) Halogens are the ascending part of the curve, And Option (D) The atomic volumes of elements in a period initially decrease and then increase are the correct answers.
So, the correct answer is “Option D”.
Note: Noble gases like Ne, Ar, Kr, which are another group of elements with similar properties, are placed just before alkali metals in every curve and are located in the ascending part of the curve along with halogens.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




