
According to Lewis dot structure the number of bond around central atom is greater than four for which of the following anion:
(A) $C{{O}_{3}}^{-2}$
(B) $N{{O}_{3}}^{-}$
(C) $C{{O}_{4}}^{-3}$
(D) none of the above
Answer
576.9k+ views
Hint: Lewis structure is drawn by taking valence electrons in account. The valence electrons are drawn around the atom, and sharing of electrons between the atoms is shown. Single bonds have two electrons shared and double bonds have 4 electrons shared between.
Complete step by step answer: Lewis structure, also known as Lewis Dot diagram, lewis dot structures, electron dot structures or lewis electron dot structures, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced this concept. Now we will draw the lewis structure of each of the options given, to know which option has more than four bonds around it. The structure of $C{{O}_{3}}^{-2}$ is as follows,
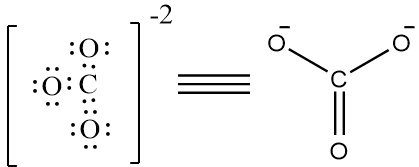
There are 4 bonds present. Therefore, it is not the correct option. The structure of $N{{O}_{3}}^{-}$ is as follows,
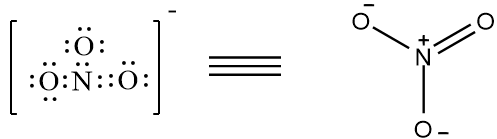
The compound has 4 bonds present. Therefore it is not the correct option. The compound $C{{O}_{4}}^{-3}$ does not exist, as carbon can’t have more than 4 bonds. So, none of the above given options have greater than four bonds around the central metal atom.
Hence, the correct answer is the D option.
Note: Lewis structure shows each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Complete step by step answer: Lewis structure, also known as Lewis Dot diagram, lewis dot structures, electron dot structures or lewis electron dot structures, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced this concept. Now we will draw the lewis structure of each of the options given, to know which option has more than four bonds around it. The structure of $C{{O}_{3}}^{-2}$ is as follows,
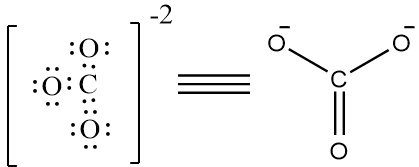
There are 4 bonds present. Therefore, it is not the correct option. The structure of $N{{O}_{3}}^{-}$ is as follows,
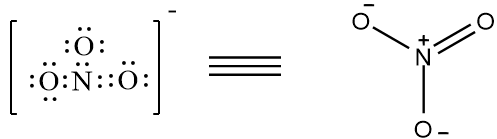
The compound has 4 bonds present. Therefore it is not the correct option. The compound $C{{O}_{4}}^{-3}$ does not exist, as carbon can’t have more than 4 bonds. So, none of the above given options have greater than four bonds around the central metal atom.
Hence, the correct answer is the D option.
Note: Lewis structure shows each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




